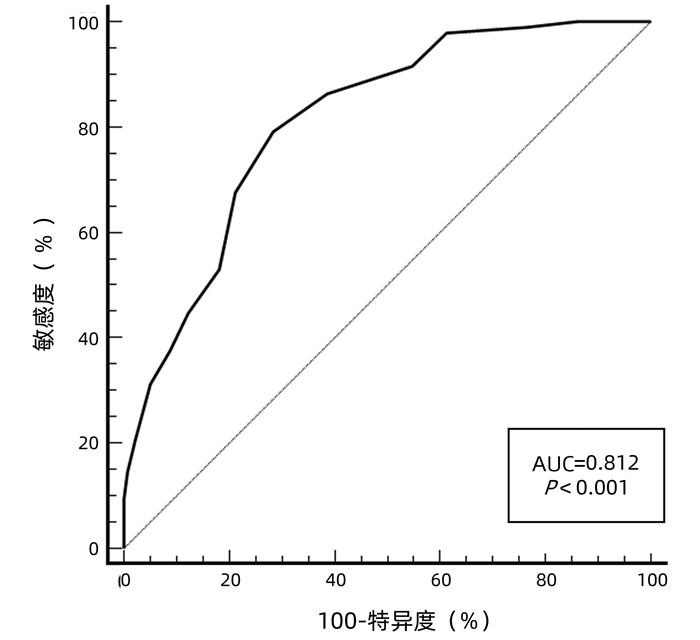
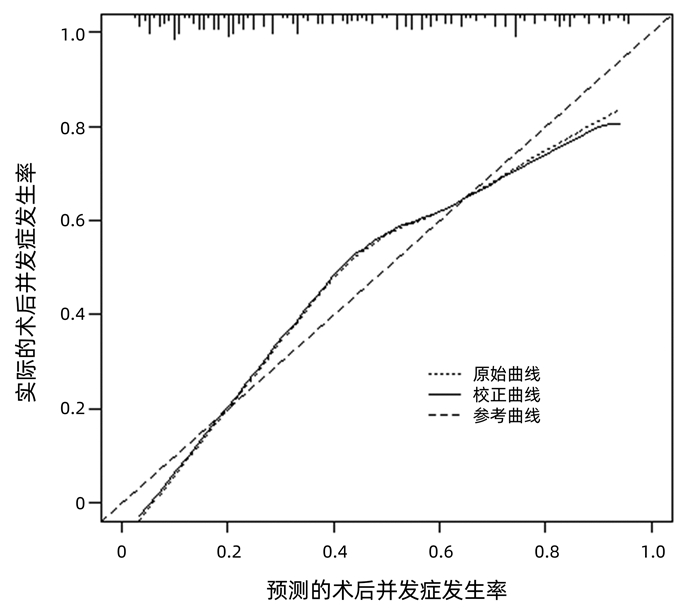
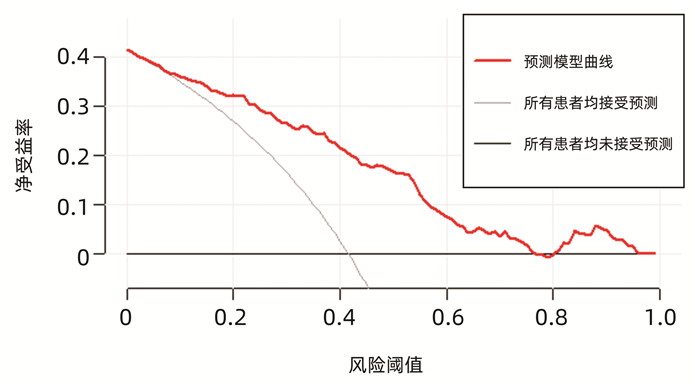
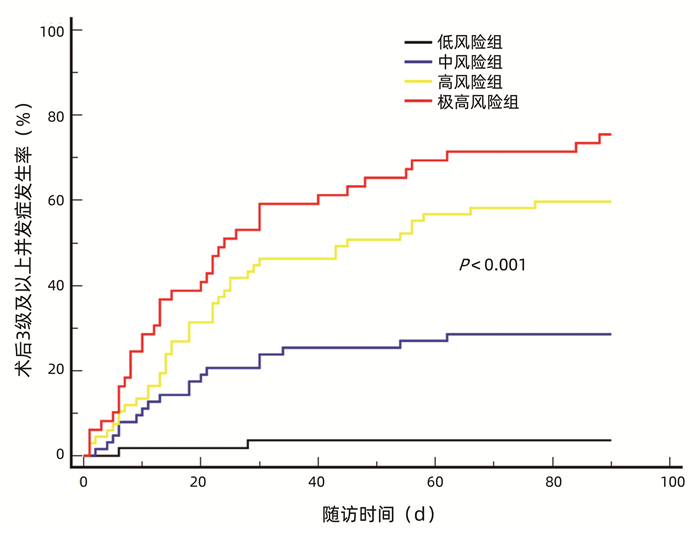
| [1] |
HOU JC, ZHENG H, QIANG Z, et al. Impact of psoas muscle index on early postoperative mortality and complications after liver transplantation[J]. Chin J Surg, 2018, 56(5): 374-378. DOI: 10.3760/cma.j.issn.0529-5815.2018.05.010. |
| [2] |
SAIMAN Y, SERPER M. Frailty and sarcopenia in patients pre- and post-liver transplant[J]. Clin Liver Dis, 2021, 25(1): 35-51. DOI: 10.1016/j.cld.2020.08.004. |
| [3] |
LEE J, JEONG WK, KIM JH, et al. Serial Observations of muscle and fat mass as prognostic factors for deceased donor liver transplantation[J]. Korean J Radiol, 2021, 22(2): 189-197. DOI: 10.3348/kjr.2019.0750. |
| [4] |
HUGUET A, LATOURNERIE M, DEBRY PH, et al. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: A retrospective cohort study[J]. Nutrition, 2018, 51-52: 73-79. DOI: 10.1016/j.nut.2018.01.008. |
| [5] |
IGNACIO de ULÍBARRI J, GONZÁLEZ-MADROÑO A, de VILLAR NG, et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population[J]. Nutr Hosp, 2005, 20(1): 38-45.
|
| [6] |
KURODA D, SAWAYAMA H, KURASHIGE J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection[J]. Gastric Cancer, 2018, 21(2): 204-212. DOI: 10.1007/s10120-017-0744-3. |
| [7] |
PRAVISANI R, MOCCHEGIANI F, ISOLA M, et al. Controlling Nutritional Status score does not predict patients' overall survival or hepatocellular carcinoma recurrence after deceased donor liver transplantation[J]. Clin Transplant, 2020, 34(3): e13786. DOI: 10.1111/ctr.13786. |
| [8] |
FUKAMI Y, SAITO T, OSAWA T, et al. Preoperative controlling nutritional status plus tumor burden score for the assessment of prognosis after curative liver resection for hepatocellular carcinoma[J]. Med Princ Pract, 2021, 30(2): 131-137. DOI: 10.1159/000514031. |
| [9] |
DINDO D, DEMARTINES N, CLAVIEN PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey[J]. Ann Surg, 2004, 240(2): 205-213. DOI: 10.1097/01.sla.0000133083.54934.ae. |
| [10] |
LYU GY. Risk factors for biliary complications after liver transplantation from donation after cardiac death[J]. J Clin Hepatol, 2015, 31(12): 2027-2030. DOI: 10.3969/j.issn.1001-5256.2015.12.009. |
| [11] |
SULLIVAN LM, MASSARO JM, SR DRB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions[J]. Stat Med, 2004, 23(10): 1631-1660. DOI: 10.1002/sim.1742. |
| [12] |
Branch of Organ Transplantation of Chinese Medical Association. Diagnosis and treatment specification for postoperative complications after liver transplantation in China (2019 edition)[J]. Organ Transpl, 2021, 12(2): 129-133. DOI: 10.3969/j.issn.1674-7445.2021.02.002. |
| [13] |
Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Gastroenterology, Chinese Medical Association. Clinical guidelines on nutrition in end-stage liver disease[J]. J Clin Hepatol, 2019, 35(6): 1222-1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010. |
| [14] |
DOIJ, MORO A, FUJIKI M, et al. Nutrition support in liver transplantation and postoperative recovery: The effects of vitamin D level and vitamin D supplementation in liver transplantation[J]. Nutrients, 2020, 12(12): 3677. DOI: 10.3390/nu12123677. |
| [15] |
TABERNA DJ, NAVAS-CARRETERO S, MARTINEZ JA. Current nutritional status assessment tools for metabolic care and clinical nutrition[J]. Curr Opin Clin Nutr Metab Care, 2019, 22(5): 323-328. DOI: 10.1097/MCO.0000000000000581. |
| [16] |
MARESCHAL J, ACHAMRAH N, NORMAN K, et al. Clinical value of muscle mass assessment in clinical conditions associated with malnutrition[J]. J Clin Med, 2019, 8(7): 1040. DOI: 10.3390/jcm8071040. |
| [17] |
CAMPOS DEL PORTILLO R, PALMA MIILA S, GARCÍA VÁQUEZ N, et al. Assessment of nutritional status in the healthcare setting in Spain[J]. Nutr Hosp, 2015, 31(Suppl 3): 196-208. DOI: 10.3305/nh.2015.31.sup3.8767. |
| [18] |
BORHOFEN SM, GERNER C, LEHMANN J, et al. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis[J]. Dig Dis Sci, 2016, 61(6): 1735-1743. DOI: 10.1007/s10620-015-4015-z. |
| [19] |
European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease[J]. J Hepatol, 2019, 70(1): 172-193. DOI: 10.1016/j.jhep.2018.06.024. |
| [20] |
ENDO K, SATO T, KAKISAKA K, et al. Calf and arm circumference as simple markers for screening sarcopenia in patients with chronic liver disease[J]. Hepatol Res, 2021, 51(2): 176-189. DOI: 10.1111/hepr.13589. |
| [21] |
YAO J, ZHOU X, YUAN L, et al. Prognostic value of the third lumbar skeletal muscle mass index in patients with liver cirrhosis and ascites[J]. Clin Nutr, 2020, 39(6): 1908-1913. DOI: 10.1016/j.clnu.2019.08.006. |
| [22] |
GADDUCCI A, COSIO S. The prognostic relevance of computed tomography-assessed skeletal muscle index and skeletal muscle radiation attenuation in patients with gynecological cancer[J]. Anticancer Res, 2021, 41(1): 9-20. DOI: 10.21873/anticanres.14747. |
| [23] |
MURRAY TÉ, WILLIAMS D, LEE MJ. Osteoporosis, obesity, and sarcopenia on abdominal CT: A review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist[J]. Abdom Radiol (NY), 2017, 42(9): 2376-2386. DOI: 10.1007/s00261-017-1124-5. |
| [24] |
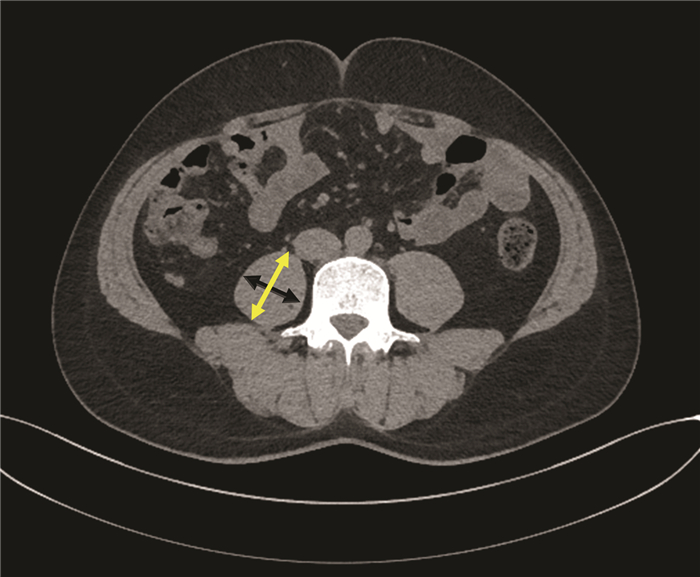
GU DH, KIM MY, SEO YS, et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis[J]. Clin Mol Hepatol, 2018, 24(3): 319-330. DOI: 10.3350/cmh.2017.0077. |
| [25] |
LIGHTFOOT A, MCARDLE A, GRIFFITHS RD. Muscle in defense[J]. Crit Care Med, 2009, 37(10 Suppl): S384-S390. DOI: 10.1097/CCM.0b013e3181b6f8a5. |
| [26] |
NEWSHOLME P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?[J]. J Nutr, 2001, 131(9 Suppl): 2515S-2522S; discussion 2523S-2524S. DOI: 10.1093/jn/131.9.2515S. |
| [27] |
ROGERI PS, GASPARINI SO, MARTINS GL, et al. Crosstalk between skeletal muscle and immune system: Which roles do IL-6 and glutamine play?[J]. Front Physiol, 2020, 11: 582258. DOI: 10.3389/fphys.2020.582258. |
| [28] |
BATATINHA H, BIONDO LA, LIRA FS, et al. Nutrients, immune system, and exercise: Where will it take us?[J]. Nutrition, 2019, 61: 151-156. DOI: 10.1016/j.nut.2018.09.019. |
| [29] |
OKAZAKI T, SUZUKAMO Y, MIYATAKE M, et al. Respiratory muscle weakness as a risk factor for pneumonia in older people[J]. Gerontology, 2021, 67(5): 581-590. DOI: 10.1159/000514007. |
| [30] |
|
| [31] |
HAN S, KIM G, LEE SK, et al. Comparison of the tolerance of hepatic ischemia/reperfusion injury in living donors: Macrosteatosis versus microsteatosis[J]. Liver Transpl, 2014, 20(7): 775-783. DOI: 10.1002/lt.23878. |














 DownLoad:
DownLoad: