| [1] |
PERINO A, DEMAGNY H, VELAZQUEZ-VILLEGAS L, et al. Molecular physiology of bile acid signaling in health, disease, and aging[J]. Physiol Rev, 2021, 101(2): 683-731. DOI: 10.1152/physrev.00049.2019. |
| [2] |
YANG F, MAO C, GUO L, et al. Structural basis of GPBAR activation and bile acid recognition[J]. Nature, 2020, 587(7834): 499-504. DOI: 10.1038/s41586-020-2569-1. |
| [3] |
KEITEL V, REINEHR R, GATSIOS P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells[J]. Hepatology, 2007, 45(3): 695-704. DOI: 10.1002/hep.21458. |
| [4] |
GUO C, CHEN WD, WANG YD. TGR5, Not only a metabolic regulator[J]. Front Physiol, 2016, 7: 646. DOI: 10.3389/fphys.2016.00646. |
| [5] |
HOLTER MM, CHIRIKJIAN MK, BRIERE DA, et al. Compound 18 improves glucose tolerance in a hepatocyte TGR5-dependent manner in mice[J]. Nutrients, 2020, 12(7): 2124. DOI: 10.3390/nu12072124. |
| [6] |
MASYUK AI, HUANG BQ, RADTKE BN, et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling[J]. Am J Physiol Gastrointest Liver Physiol, 2013, 304(11): G1013-G1024. DOI: 10.1152/ajpgi.00383.2012. |
| [7] |
NAKHI A, WONG HL, WELDY M, et al. Structural modifications that increase gut restriction of bile acid derivatives[J]. RSC Med Chem, 2021, 12(3): 394-405. DOI: 10.1039/d0md00425a. |
| [8] |
VASSILEVA G, HU W, HOOS L, et al. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice[J]. J Endocrinol, 2010, 205(3): 225-232. DOI: 10.1677/JOE-10-0009. |
| [9] |
WANG YD, CHEN WD, YU D, et al. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor κ light-chain enhancer of activated B cells (NF-κB) in mice[J]. Hepatology, 2011, 54(4): 1421-1432. DOI: 10.1002/hep.24525. |
| [10] |
BIAGIOLI M, MARCHIANÒ S, CARINO A, et al. Bile acids activated receptors in inflammatory bowel disease[J]. Cells, 2021, 10(6): 1281. DOI: 10.3390/cells10061281. |
| [11] |
YANG H, ZHOU H, ZHUANG L, et al. Plasma membrane-bound G protein-coupled bile acid receptor attenuates liver ischemia/reperfusion injury via the inhibition of toll-like receptor 4 signaling in mice[J]. Liver Transpl, 2017, 23(1): 63-74. DOI: 10.1002/lt.24628. |
| [12] |
GUO C, QI H, YU Y, et al. The G-Protein-Coupled Bile Acid Receptor Gpbar1 (TGR5) inhibits gastric inflammation through antagonizing NF-κB signaling pathway[J]. Front Pharmacol, 2015, 6: 287. DOI: 10.3389/fphar.2015.00287. |
| [13] |
SU J, ZHANG Q, QI H, et al. The G-protein-coupled bile acid receptor Gpbar1 (TGR5) protects against renal inflammation and renal cancer cell proliferation and migration through antagonizing NF-κB and STAT3 signaling pathways[J]. Oncotarget, 2017, 8(33): 54378-54387. DOI: 10.18632/oncotarget.17533. |
| [14] |
YANG H, LUO F, WEI Y, et al. TGR5 protects against cholestatic liver disease via suppressing the NF-κB pathway and activating the Nrf2/HO-1 pathway[J]. Ann Transl Med, 2021, 9(14): 1158. DOI: 10.21037/atm-21-2631. |
| [15] |
ZHAO X, LI H, LYU S, et al. Single-cell transcriptomics reveals heterogeneous progression and EGFR activation in pancreatic adenosquamous carcinoma[J]. Int J Biol Sci, 2021, 17(10): 2590-2605. DOI: 10.7150/ijbs.58886. |
| [16] |
REICH M, DEUTSCHMANN K, SOMMERFELD A, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro[J]. Gut, 2016, 65(3): 487-501. DOI: 10.1136/gutjnl-2015-309458. |
| [17] |
LIU X, CHEN B, YOU W, et al. The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer[J]. Cancer Lett, 2018, 412: 194-207. DOI: 10.1016/j.canlet.2017.10.017. |
| [18] |
PATHAK P, LIU H, BOEHME S, et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism[J]. J Biol Chem, 2017, 292(26): 11055-11069. DOI: 10.1074/jbc.M117.784322. |
| [19] |
BIDAULT-JOURDAINNE V, MERLEN G, GLÉNISSON M, et al. TGR5 controls bile acid composition and gallbladder function to protect the liver from bile acid overload[J]. JHEP Rep, 2021, 3(2): 100214. DOI: 10.1016/j.jhepr.2020.100214. |
| [20] |
PÉAN N, DOIGNON I, GARCIN I, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice[J]. Hepatology, 2013, 58(4): 1451-1460. DOI: 10.1002/hep.26463. |
| [21] |
DONEPUDI AC, BOEHME S, LI F, et al. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice[J]. Hepatology, 2017, 65(3): 813-827. DOI: 10.1002/hep.28707. |
| [22] |
KEITEL V, CUPISTI K, ULLMER C, et al. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders[J]. Hepatology, 2009, 50(3): 861-870. DOI: 10.1002/hep.23032. |
| [23] |
KEITEL V, REICH M, HÄUSSINGER D. TGR5: pathogenetic role and/or therapeutic target in fibrosing cholangitis?[J]. Clin Rev Allergy Immunol, 2015, 48(2-3): 218-225. DOI: 10.1007/s12016-014-8443-x. |
| [24] |
LI T, HOLMSTROM SR, KIR S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling[J]. Mol Endocrinol, 2011, 25(6): 1066-1071. DOI: 10.1210/me.2010-0460. |
| [25] |
SHI Y, SU W, ZHANG L, et al. TGR5 regulates macrophage inflammation in nonalcoholic steatohepatitis by modulating NLRP3 inflammasome activation[J]. Front Immunol, 2020, 11: 609060. DOI: 10.3389/fimmu.2020.609060. |
| [26] |
KANG JH, KIM M, YIM M. FXR/TGR5 mediates inflammasome activation and host resistance to bacterial infection[J]. Biochem Biophys Rep, 2021, 27: 101051. DOI: 10.1016/j.bbrep.2021.101051. |
| [27] |
FRANKE A, BALSCHUN T, KARLSEN TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility[J]. Nat Genet, 2008, 40(11): 1319-1323. DOI: 10.1038/ng.221. |
| [28] |
KARLSEN TH, FRANKE A, MELUM E, et al. Genome-wide association analysis in primary sclerosing cholangitis[J]. Gastroenterology, 2010, 138(3): 1102-1111. DOI: 10.1053/j.gastro.2009.11.046. |
| [29] |
DEUTSCHMANN K, REICH M, KLINDT C, et al. Bile acid receptors in the biliary tree: TGR5 in physiology and disease[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864(4 Pt B): 1319-1325. DOI: 10.1016/j.bbadis.2017.08.021. |
| [30] |
REICH M, SPOMER L, KLINDT C, et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis[J]. J Hepatol, 2021, 75(3): 634-646. DOI: 10.1016/j.jhep.2021.03.029. |
| [31] |
ZHANG R, MA WQ, FU MJ, et al. Overview of bile acid signaling in the cardiovascular system[J]. World J Clin Cases, 2021, 9(2): 308-320. DOI: 10.12998/wjcc.v9.i2.308. |
| [32] |
FRYER RM, NG KJ, NODOP MAZUREK SG, et al. G protein-coupled bile acid receptor 1 stimulation mediates arterial vasodilation through a K(Ca)1.1 (BK(Ca))-dependent mechanism[J]. J Pharmacol Exp Ther, 2014, 348(3): 421-431. DOI: 10.1124/jpet.113.210005. |
| [33] |
YUSTA B, MATTHEWS D, FLOCK GB, et al. Glucagon-like peptide-2 promotes gallbladder refilling via a TGR5-independent, GLP-2R-dependent pathway[J]. Mol Metab, 2017, 6(6): 503-511. DOI: 10.1016/j.molmet.2017.03.006. |
| [34] |
ERICE O, LABIANO I, ARBELAIZ A, et al. Differential effects of FXR or TGR5 activation in cholangiocarcinoma progression[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864(4 Pt B): 1335-1344. DOI: 10.1016/j.bbadis.2017.08.016. |
| [35] |
XU L, HAUSMANN M, DIETMAIER W, et al. Expression of growth factor receptors and targeting of EGFR in cholangiocarcinoma cell lines[J]. BMC Cancer, 2010, 10: 302. DOI: 10.1186/1471-2407-10-302. |
| [36] |
LAVOIE B, BALEMBA OB, GODFREY C, et al. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels[J]. J Physiol, 2010, 588(Pt 17): 3295-3305. DOI: 10.1113/jphysiol.2010.192146. |
| [37] |
BRIERE DA, RUAN X, CHENG CC, et al. Novel small molecule agonist of TGR5 possesses anti-diabetic effects but causes gallbladder filling in mice[J]. PLoS One, 2015, 10(8): e0136873. DOI: 10.1371/journal.pone.0136873. |
| [38] |
VASSILEVA G, GOLOVKO A, MARKOWITZ L, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation[J]. Biochem J, 2006, 398(3): 423-430. DOI: 10.1042/BJ20060537. |
| [39] |
MASYUK TV, MASYUK AI, LORENZO PISARELLO M, et al. TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Gαs signaling[J]. Hepatology, 2017, 66(4): 1197-1218. DOI: 10.1002/hep.29284. |
| [40] |
LARUSSO NF, MASYUK TV, HOGAN MC. Polycystic liver disease: The benefits of targeting cAMP[J]. Clin Gastroenterol Hepatol, 2016, 14(7): 1031-1034. DOI: 10.1016/j.cgh.2016.03.008. |
| [41] |
SUSSMAN CR, WANG X, CHEBIB FT, et al. Modulation of polycystic kidney disease by G-protein coupled receptors and cyclic AMP signaling[J]. Cell Signal, 2020, 72: 109649. DOI: 10.1016/j.cellsig.2020.109649. |



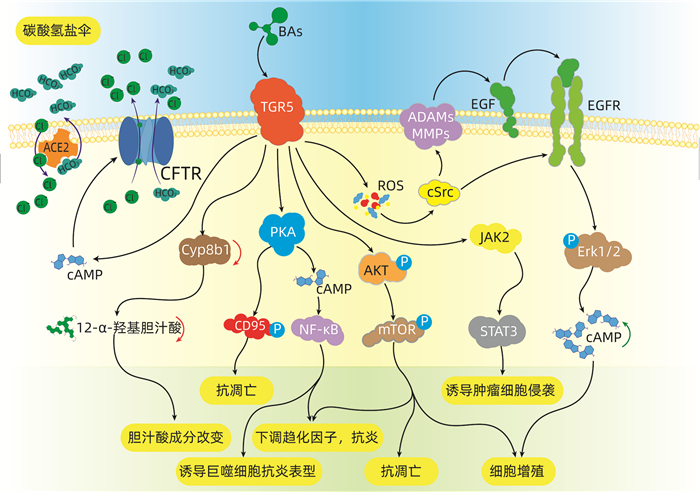




 DownLoad:
DownLoad: