| [1] |
Branch of Hepatobiliary Diseases, China Association of Chinese Medicine; Branch of Chinese Patent Medicine, China Association of Chinese Medicine. Guideline for diagnosis and treatment of herb-induced liver injury[J]. J Clin Hepatol, 2016, 32(5): 835-843. DOI: 10.3969/j.issn.1001-5256.2016.05.003. |
| [2] |
AN XD, WEI Y, LIAN FM. Clinical application and dosage of fleeceflower root[J]. J Changchun Univ Chin Med, 2020, 36(2): 219-221. DOI: 10.13463/j.cnki.cczyy.2020.02.005. |
| [3] |
CAO YN, WANG NN, ZHOU GQ, et al. Analysis of influencing factors of clinical outcome in 186 patients with the drug-in-duced liver injury with positive autoantibodies[J]. Chin J Integr Tradit West Med Liver Dis, 2021, 31(1): 26-29, 33. DOI: 10.3969/j.issn.1005-0264.2021.01.007. |
| [4] |
YIN JY. Brief analysis of clinical value of detecting autoimmune antibody in patients with drug-induced liver injury[J]. China Prac Med, 2021, 16(7): 77-79. DOI: 10.14163/j.cnki.11-5547/r.2021.07.031. |
| [5] |
Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002. |
| [6] |
Chinese Society of Hepatology, Chinese Medical Association. Guidelines on the diagnosis and management of autoimmune hepatitis (2021)[J]. J Clin Hepatol, 2022, 38(1): 42-49. DOI: 10.3760/cma.j.cn112138-20211112-00796. |
| [7] |
SRIVASTAVA A, MAGGS JL, ANTOINE DJ, et al. Role of reactive metabolites in drug-induced hepatotoxicity[J]. Handb Exp Pharmacol, 2010, 196: 165-194. DOI: 10.1007/978-3-642-00663-0_7. |
| [8] |
VINKEN M, MAES M, VANHAECKE T, et al. Drug-induced liver injury: mechanisms, types and biomarkers[J]. Curr Med Chem, 2013, 20(24): 3011-3021. DOI: 10.2174/0929867311320240006. |
| [9] |
FONTANA RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives[J]. Gastroenterology, 2014, 146(4): 914-928. DOI: 10.1053/j.gastro.2013.12.032. |
| [10] |
REN MX, CHEN J, HUANG CY, et al. The clinical and pathological characteristics of drug-induced liver injury accompanied by autoimmune phenomena[J]. J China-Japan Friendship Hosp, 2018, 32(5): 279-283. DOI: 10.3969/j.issn.1001-0025.2018.05.006. |
| [11] |
|
| [12] |
STINE JG, CHALASANI N. Chronic liver injury induced by drugs: a systematic review[J]. Liver Int, 2015, 35(11): 2343-2353. DOI: 10.1111/liv.12958. |
| [13] |
MENG YK, LI CY, LI RY, et al. Cis-stilbene glucoside in Polygonum multiflorum induces immunological idiosyncratic hepatotoxicity in LPS-treated rats by suppressing PPAR-γ[J]. Acta Pharmacol Sin, 2017, 38(10): 1340-1352. DOI: 10.1038/aps.2017.32. |
| [14] |
TENG SS, SUN Z, QIU Y, et al. Investigation, optimization and evaluation of traditional Chinese medicine processing technology of nine steaming and nine drying[J]. J Changchun Univ Chin Med, 2022, 38(1): 109-113. DOI: 10.13463/j.cnki.cczyy.2022.01.026. |



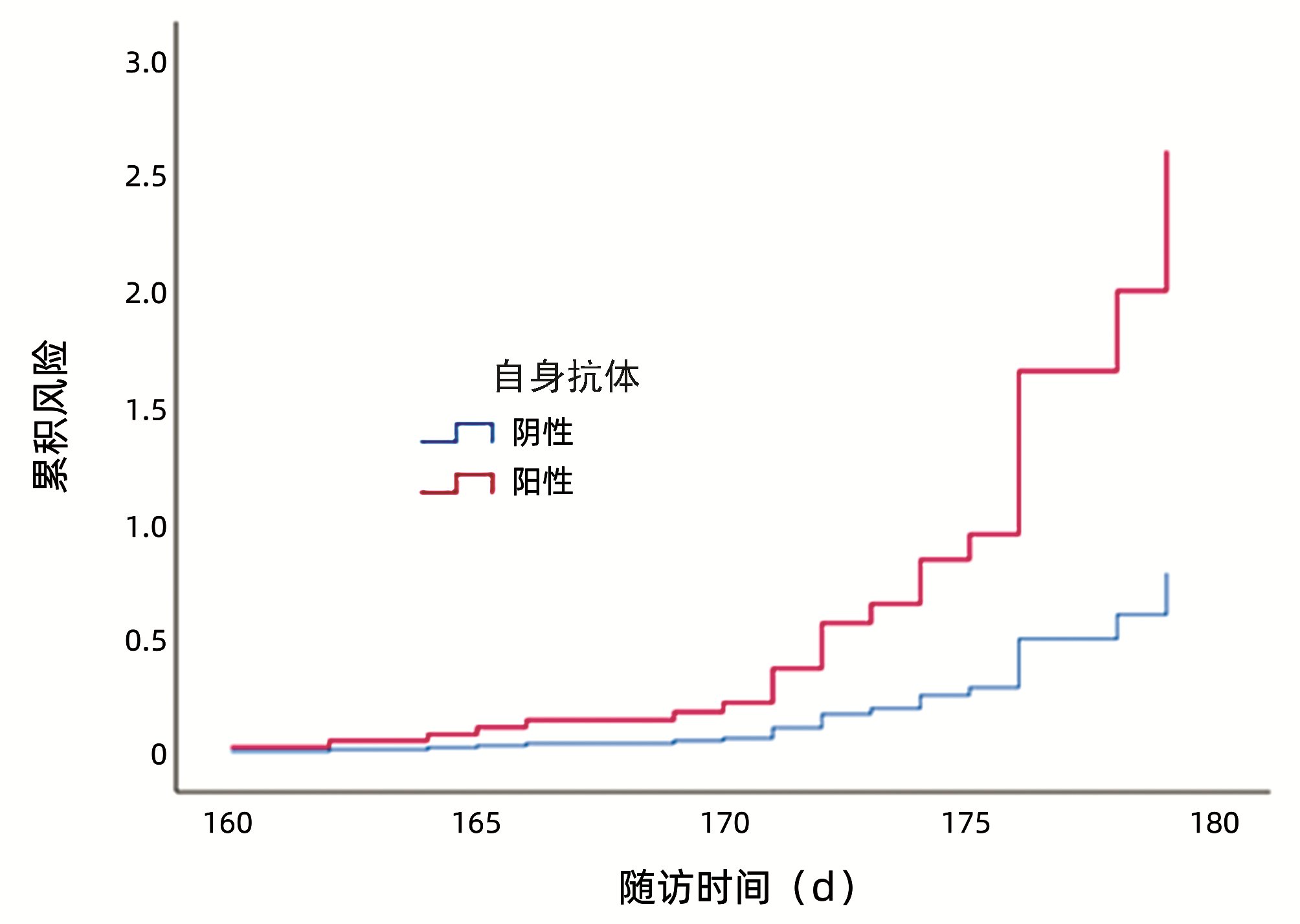




 DownLoad:
DownLoad: