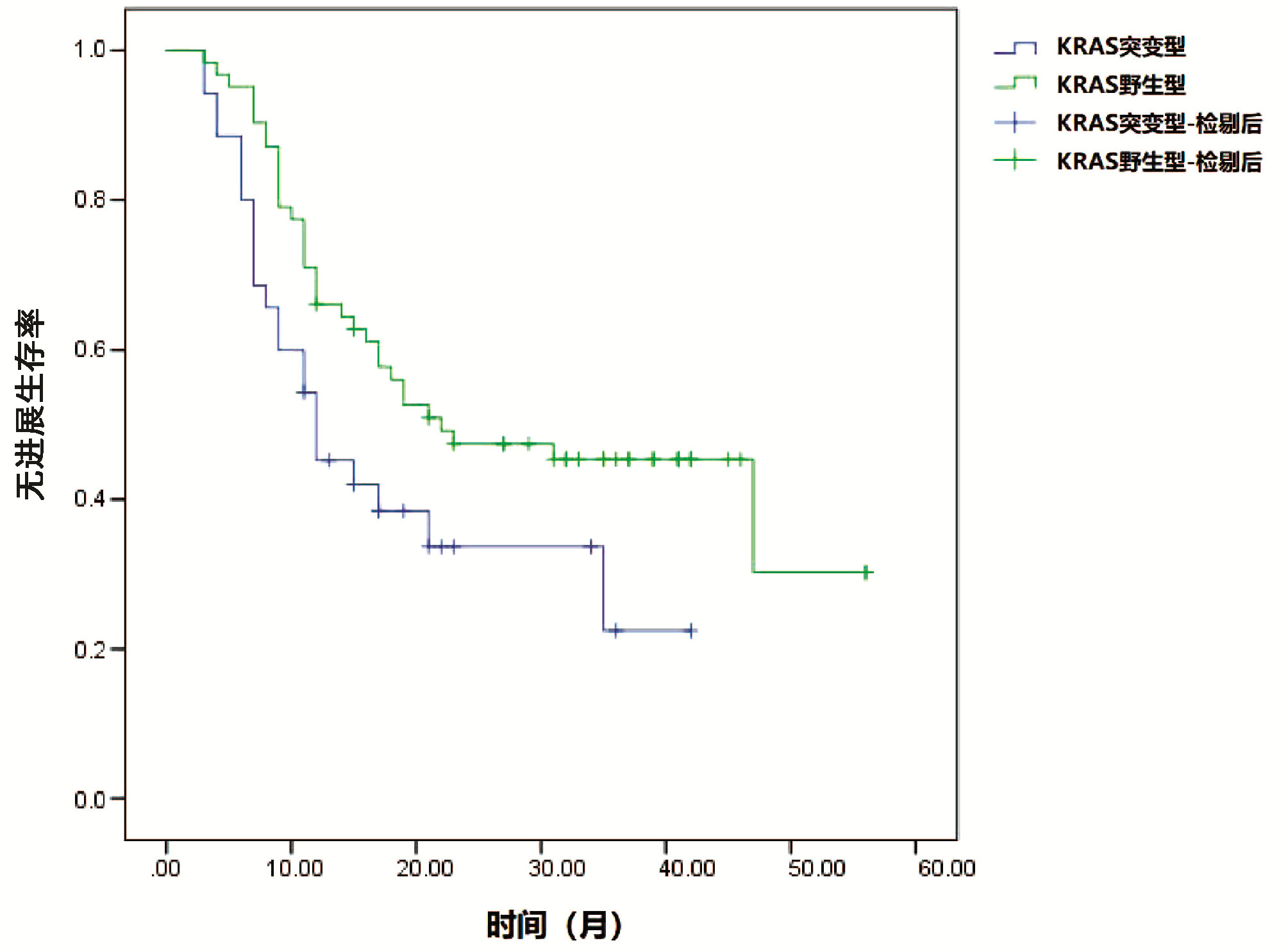
| [1] |
LI X, RAMADORI P, PFISTER D, et al. The immunological and metabolic landscape in primary and metastatic liver cancer[J]. Nat Rev Cancer, 2021, 21(9): 541-557. DOI: 10.1038/s41568-021-00383-9. |
| [2] |
|
| [3] |
LIU CX, CHANG K, NA WL, et al. Role of differential expression and regulatory mechanism of miR-152-3 p target proteins in the recurrence of hepa-tocellular carcinoma[J]. J Clin Hepatol, 2021, 37(2): 364-369. DOI: 10.3969/j.issn.1001-5256.2021.02.023. |
| [4] |
BLACKBURN H, WEST S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver cancer[J]. Cancer Nurs, 2016, 39(5): E1-E18. DOI: 10.1097/NCC.0000000000000302. |
| [5] |
CHENG YR, YAN D, YANG JD, et al. Effect of hepatic artery chemoembolization combined with sorafenib in the treatment of primary liver cancer and its influence on patients' immune function[J]. Clin J Med Offic, 2021, 49(3): 290-291. DOI: 10.16680/j.1671-3826.2021.03.16. |
| [6] |
RECK M, MOK T, NISHIO M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial[J]. Lancet Respir Med, 2019, 7(5): 387-401. DOI: 10.1016/S2213-2600(19)30084-0. |
| [7] |
CUI FQ, LI T, WANG Z, et al. A pilot study on cetuximab and KRAS gene mutation in treatment of patients with primary liver can-cer[J]. J Prac Hepatol, 2019, 22(4): 565-568. DOI: 10.3969/j.issn.1672-5069.2019.04.029. |
| [8] |
PAN CF, ZHAO SK, LI Y, et al. Mutation study of related genes in non-small cell lung cancer drug targeting sites[J]. Hainan Med J, 2018, 29(19): 2696-2698. DOI: 10.3969/j.issn.1003-6350.2018.19.009. |
| [9] |
WU ST. Application of contrast-enhanced CT Scan in curative effect evaluation of primary hepatocellular carcinoma after TACE[J]. Chin J CT and MRI, 2022, 20(3): 91-93. DOI: 10.3969/j.issn.1672-5131.2022.03.030. |
| [10] |
KIM D, LEE JH, MOON H, et al. Development and evaluation of an ultrasound-triggered microbubble combined transarterial chemoembolization (TACE) formulation on rabbit VX2 liver cancer model[J]. Theranostics, 2021, 11(1): 79-92. DOI: 10.7150/thno.45348. |
| [11] |
CHEN XW, JIANG JW, LIN FH. Longdan xiegan decoction in the treatment of embolism syndrome after interventional therapy on primary hepatocellular carcinoma[J]. J Nangjing Univ Tradit Chin Med, 2016, 32(3): 224-228. DOI: 10.14148/j.issn.1672-0482.2016.0224. |
| [12] |
YOU GM, JING BL, PAN Q, et al. Compression hemostasis with Shunlin arterial hemostatic dressing for patients with hepatocellular carcinoma after transcatheter arterial chemoembolization: its clinical application and efficacy[J]. J Intervent Radiol, 2021, 30(5): 519-522. DOI: 10.3969/j.issn.1008-794X.2021.05.021. |
| [13] |
LUO Y, JIANG Y. Comparison of efficiency of TACE plus HIFU and TACE alone on patients with primary liver cancer[J]. J Coll Physicians Surg Pak, 2019, 29(5): 414-417. DOI: 10.29271/jcpsp.2019.05.414. |
| [14] |
VOGL TJ, MARKO C, LANGENBACH MC, et al. Transarterial chemoembolization of colorectal cancer liver metastasis: improved tumor response by DSM-TACE versus conventional TACE, a prospective, randomized, single-center trial[J]. Eur Radiol, 2021, 31(4): 2242-2251. DOI: 10.1007/s00330-020-07253-2. |
| [15] |
ZHANG X, ZHOU J, ZHU DD, et al. CalliSpheres ® drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study[J]. Clin Transl Oncol, 2019, 21(2): 167-177. DOI: 10.1007/s12094-018-1902-8. |
| [16] |
AARTS BM, MUÑOZ F, WILDIERS H, et al. Intra-arterial therapies for liver metastatic breast cancer: a systematic review and meta-analysis[J]. Cardiovasc Intervent Radiol, 2021, 44(12): 1868-1882. DOI: 10.1007/s00270-021-02906-1. |
| [17] |
SUN ZQ, JIANG CY, LI BP, et al. Clinical efficacy and adverse reactions of TACE combined with different doses of apatinib in the treatment of advanced liver cancer[J]. Chin J Gerontol, 2022, 42(3): 557-560. DOI: 10.3969/j.issn.1005-9202.2022.03.014. |
| [18] |
HUANG CS, YU W, WANG Q, et al. Clinical efficacy of sorafenib and TACE for primary liver cancer and its effect on bFGF and VEGF level[J]. Pract J Cancer, 2017, 32(6): 943-945. DOI: 10.3969/j.issn.1001-5930.2017.06.021 |
| [19] |
YE PL, JIA HY, PENG L. Mechanism of action of GP73 in the regulation of liver cancer: An analysis based on transcriptome sequencing[J]. J Clin Hepatol, 2021, 37(8): 1861-1866. DOI: 10.3969/j.issn.1001-5256.2021.08.022. |
| [20] |
QIN L, ZHAN Z, WEI C, et al. Hsa-circRNA-G004213 promotes cisplatin sensitivity by regulating miR-513b-5p/PRPF39 in liver cancer[J]. Mol Med Rep, 2021, 23(6): 421. DOI: 10.3892/mmr.2021.12060. |
| [21] |
YIN XL, XU Q. The clinical significance of KRAS and BRAF oncogene mutations in hepatocellular carcinoma[J]. J Modern Oncology, 2016, 24(15): 2419-2422. DOI: 10.3969/j.issn.1672-4992.2016.15.022. |



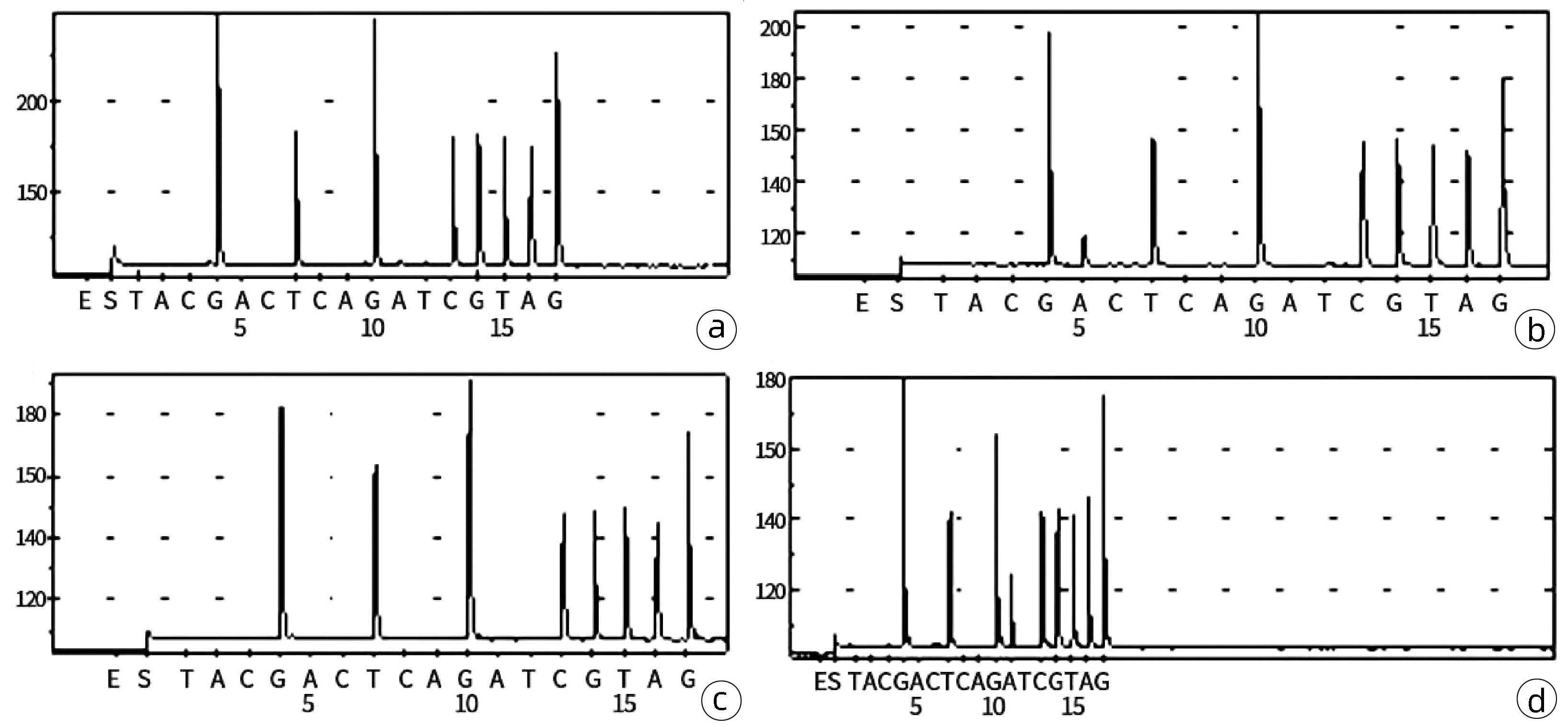




 DownLoad:
DownLoad: