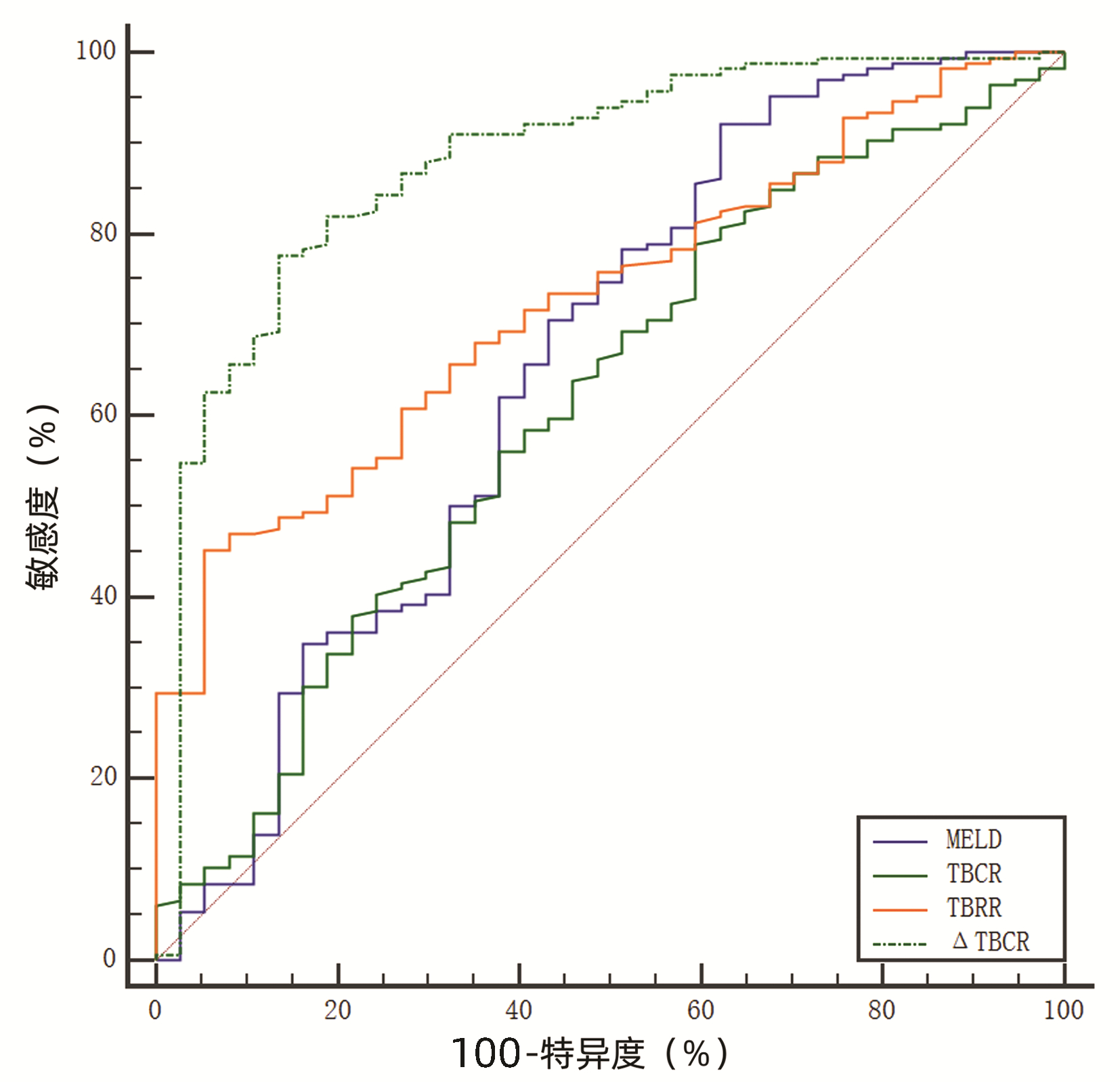
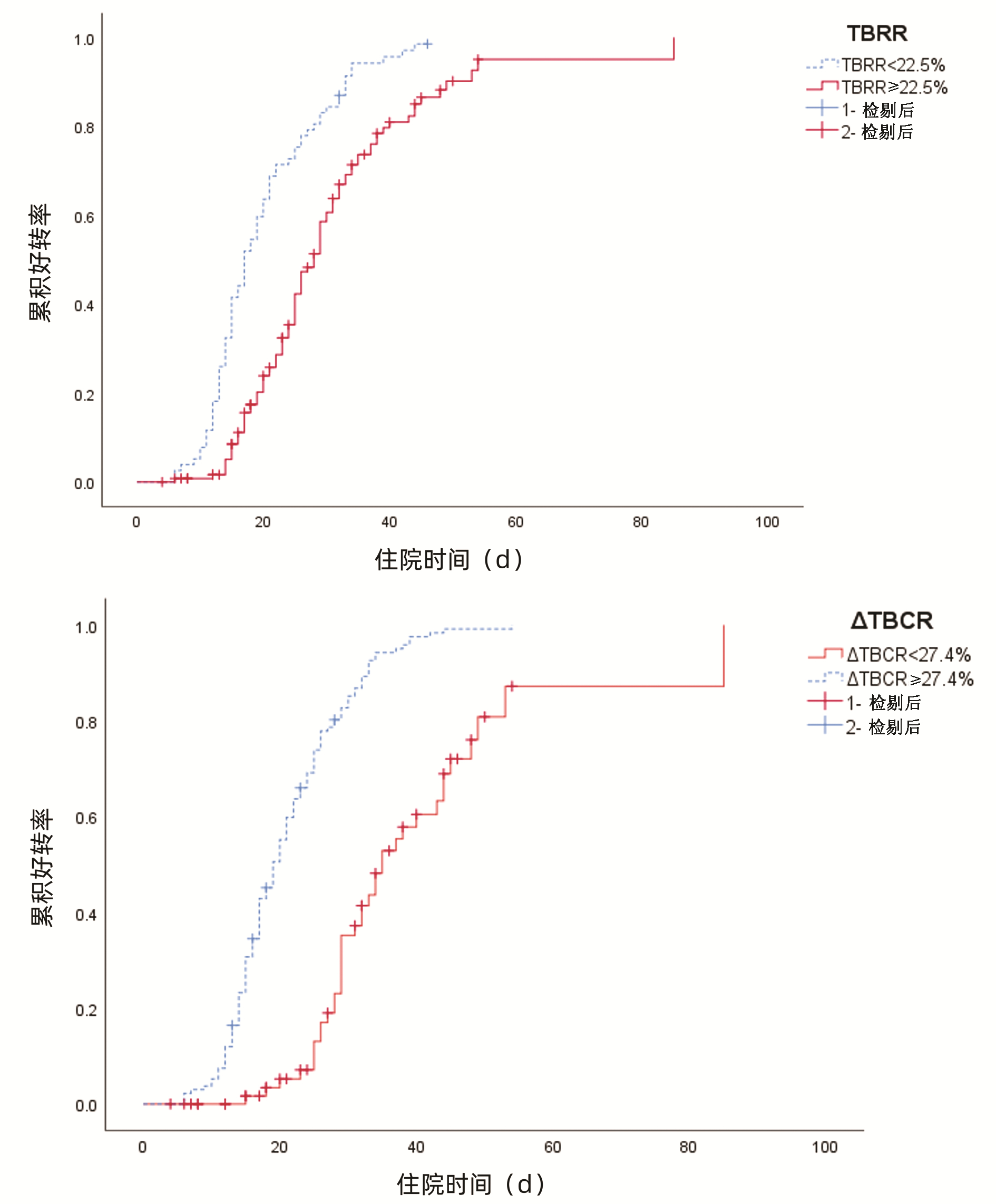
| [1] |
DEVARBHAVI H, AITHAL G, TREEPRASERTSUK S, et al. Drug-induced liver injury: Asia Pacific Association of Study of Liver consensus guidelines[J]. Hepatol Int, 2021, 15(2): 258-282. DOI: 10.1007/s12072-021-10144-3. |
| [2] |
Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury[J]. J Clin Hepatol, 2015, 31(11): 1752-1769. DOI: 10.3969/j.issn.1001-5256.2015.11.002. |
| [3] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases. Guideline for nonbioartificial liver support systems in treatment of liver failure: 2016 update[J]. Chin J Clin Infect Dis, 2016, 9(2): 97-103. DOI: 10.3760/cma.j.issn. 1674-2397.2016.02.001.
中华医学会感染病学分会肝衰竭与人工肝学组. 非生物型人工肝治疗肝衰竭指南(2016年版)[J]. 中华临床感染病杂志, 2016, 9(2): 97-103. DOI: 10.3760/cma. j.issn.1674-2397.2016.02.001.
|
| [4] |
Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Expert consensus on clinical application of artificial liver and blood purification (2022 edition)[J]. J Clin Hepatol, 2022, 38(4): 767-775. DOI: 10.3969/j.issn.1001-5256.2022.04.007. |
| [5] |
SHAKIL AO, KRAMER D, MAZARIEGOS GV, et al. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria[J]. Liver Transpl, 2000, 6(2): 163-169. DOI: 10.1002/lt.500060218. |
| [6] |
SILBERHUMER GR, HETZ H, RASOUL-ROCKENSCHAUB S, et al. Is MELD score sufficient to predict not only death on waiting list, but also post-transplant survival?[J]. Transpl Int, 2006, 19(4): 275-281. DOI: 10.1111/j.1432-2277.2006.00250.x. |
| [7] |
WANG ZC, SHAO JG, GU EL. Short term prediction of rebound rate of total bilirubin in patients with chronic subacute liver failure treated with artificial liver[J]. Chin J Infect Dis, 2013, 31(11): 678-680. DOI: 10.3760/cma.j.issn.1000-6680.2013.11.009. |
| [8] |
XIN KF, LI M, LI SS, et al. The role of TBARR, TBRR and TBCR in evaluating the prognosis of patients with chronic acute liver failure after plasma exchange therapy[J]. Shandong Med J, 2018, 58(25): 44-46. DOI: 10.3969/j.issn.1002-266X.2018.25.012. |
| [9] |
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35(1): 38-44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. |
| [10] |
CHALASANI NP, MADDUR H, RUSSO MW, et al. ACG clinical guideline: diagnosis and management of idiosyncratic drug-induced liver injury[J]. Am J Gastroenterol, 2021, 116(5): 878-898. DOI: 10.14309/ajg. 0000000000001259.
|
| [11] |
FORMAN LM, LUCEY MR. Predicting the prognosis of chronic liver disease: an evolution from child to MELD. Mayo end-stage liver disease[J]. Hepatology, 2001, 33(2): 473-475. DOI: 10.1053/jhep.2001.22481. |
| [12] |
CHEN M, SUZUKI A, BORLAK J, et al. Drug-induced liver injury: Interactions between drug properties and host factors[J]. J Hepatol, 2015, 63(2): 503-514. DOI: 10.1016/j.jhep.2015.04.016. |
| [13] |
TIAN B, LI F, DENG BC. Clinical effect of artificial liver support system in treatment of drug-induced liver failure: A meta-analysis [J]. J Clin Hepatol, 2020, 36(4): 823-828. DOI: 10.3969/j.issn.1001-5256.2020.04.023. 田冰, 李范, 邓宝成. 人工肝支持系统治疗药物性肝衰竭临床效果的Meta分析[J]. 临床肝胆病杂志, 2020, 36(4): 823-828. DOI: 10.3969/j.issn. 1001-5256.2020.04.023.
|
| [14] |
|
| [15] |
WU B, DU LY, MA YJ, et al. Effects of different combinations of artificial liver support system on efficacy and inflammatory indexes of patients with hepatitis B virus-related acute-on-chronic liver failure in early and middle stages[J/CD]. Chin J Liver Dis (Electronic Version), 2021, 13(1): 32-38. DOI: 10.3969/j.issn.1674-7380.2021.01.006.
吴蓓, 杜凌遥, 马元吉, 等. 不同组合人工肝支持系统治疗乙型肝炎病毒相关早、中期慢加急性肝衰竭患者的疗效及对炎症指标的影响[J/CD]. 中国肝脏病杂志(电子版), 2021, 13(1): 32-38. DOI: 10.3969/j.issn.1674-7380.2021.01.006.
|
| [16] |
MORALES-ARRÁEZ D, VENTURA-COTS M, ALTAMIRANO J, et al. The MELD score is superior to the maddrey discriminant function score to predict short-term mortality in alcohol-associated hepatitis: A global study[J]. Am J Gastroenterol, 2022, 117(2): 301-310. DOI: 10.14309/ajg.0000000000001596. |
| [17] |
KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33(2): 464-470. DOI: 10.1053/jhep.2001.22172. |
| [18] |
ZHANG YF, YU WY, CHEN DL, et al. The prognosis of acute (subacute) hepatic failure with hepatic encephalopathy treated with artificial liver[J/CD]. Prac J Organ Transplant, 2020, 8(4): 252-255. DOI: 10.3969/j.issn.2095-5332.2020.04.004.
张叶凡, 于万有, 陈冬玲, 等. 人工肝治疗急(亚急)性肝衰竭合并肝性脑病的中短期预后评估[J/CD]. 实用器官移植电子杂志, 2020, 8(4): 252-255. DOI: 10.3969/j.issn.2095-5332.2020.04.004.
|



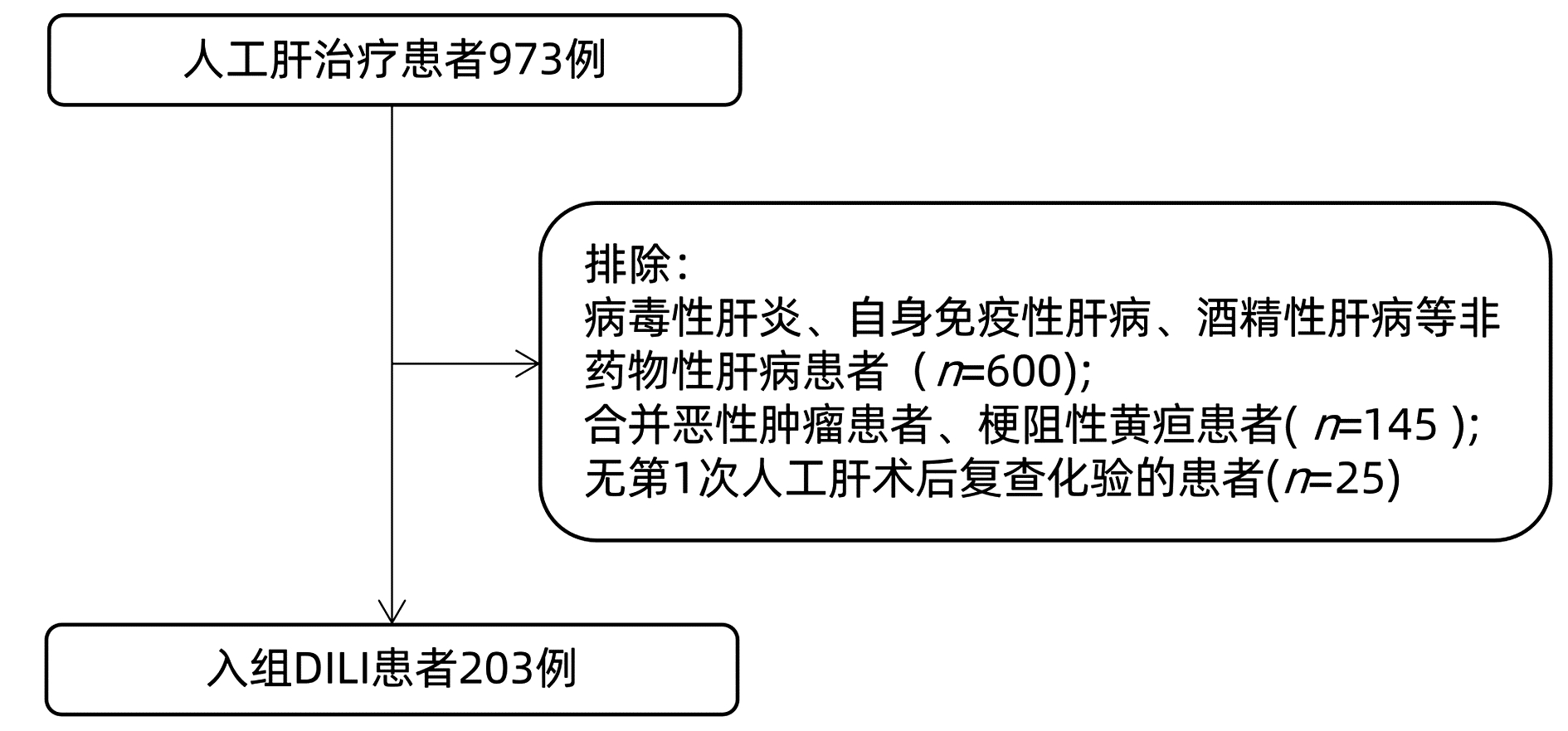




 DownLoad:
DownLoad: