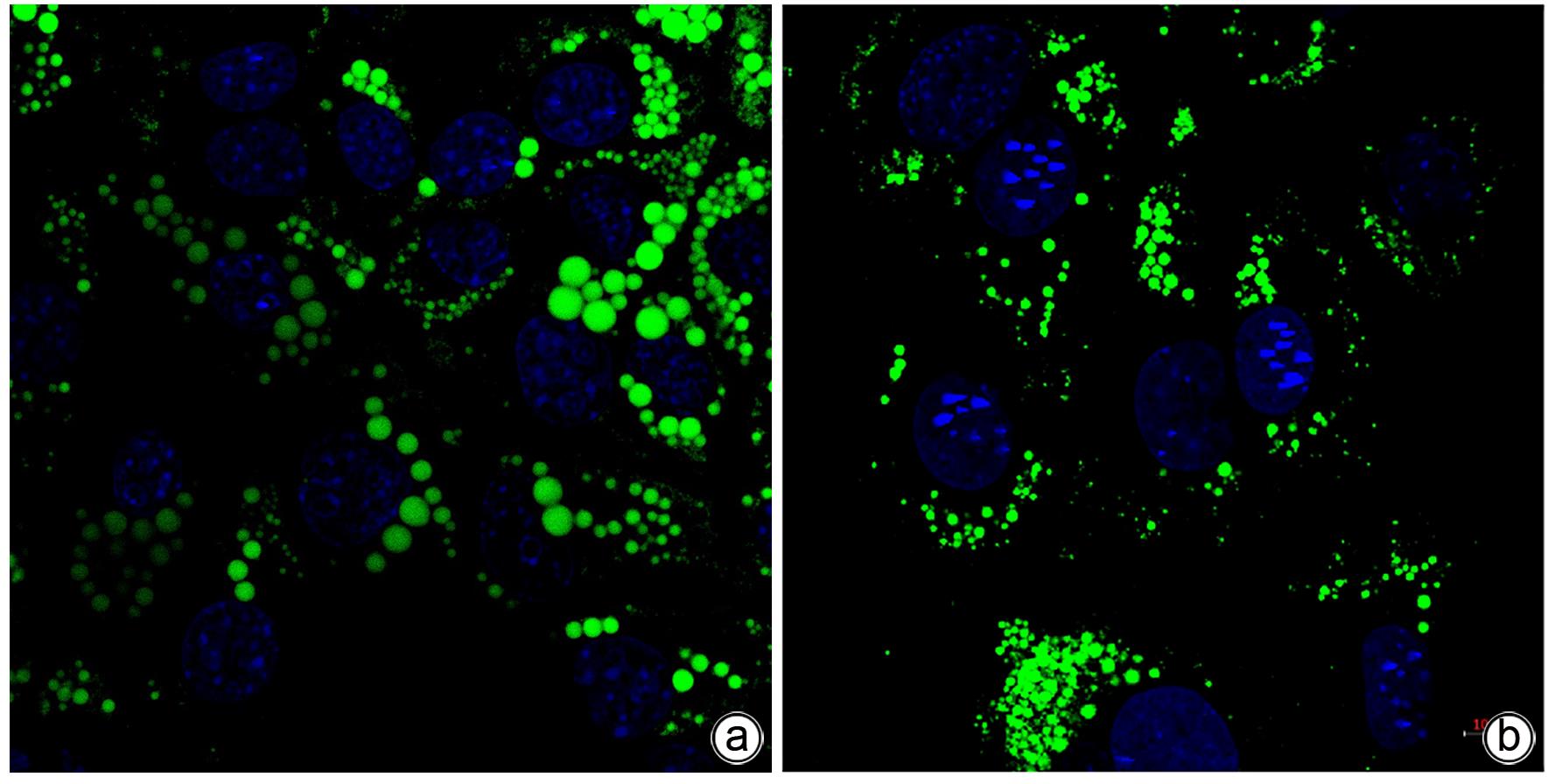
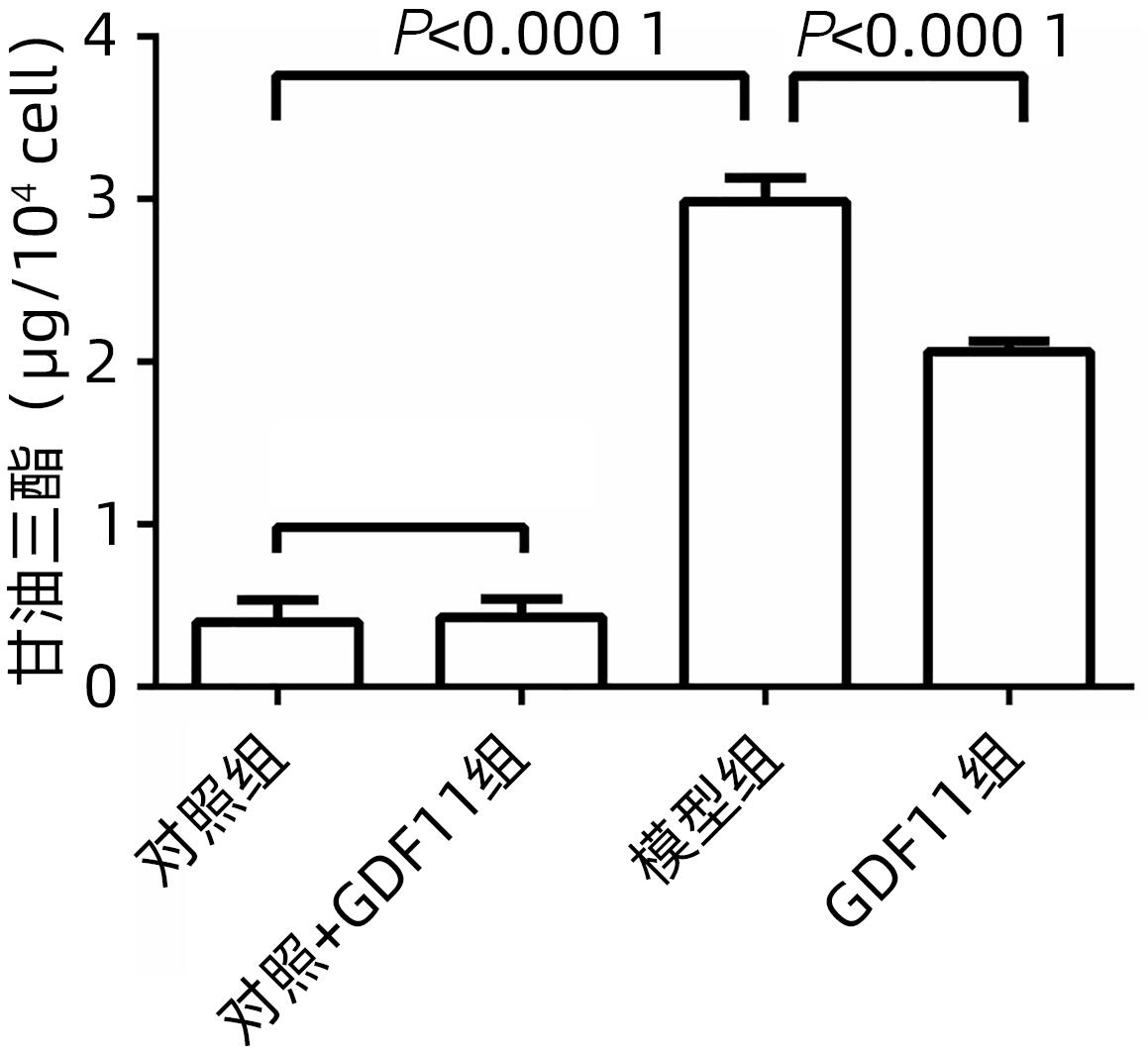
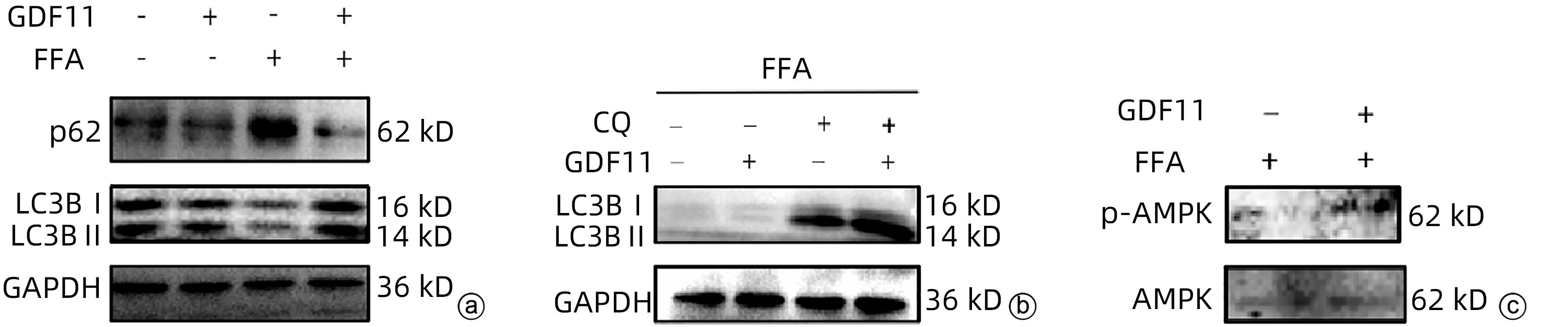
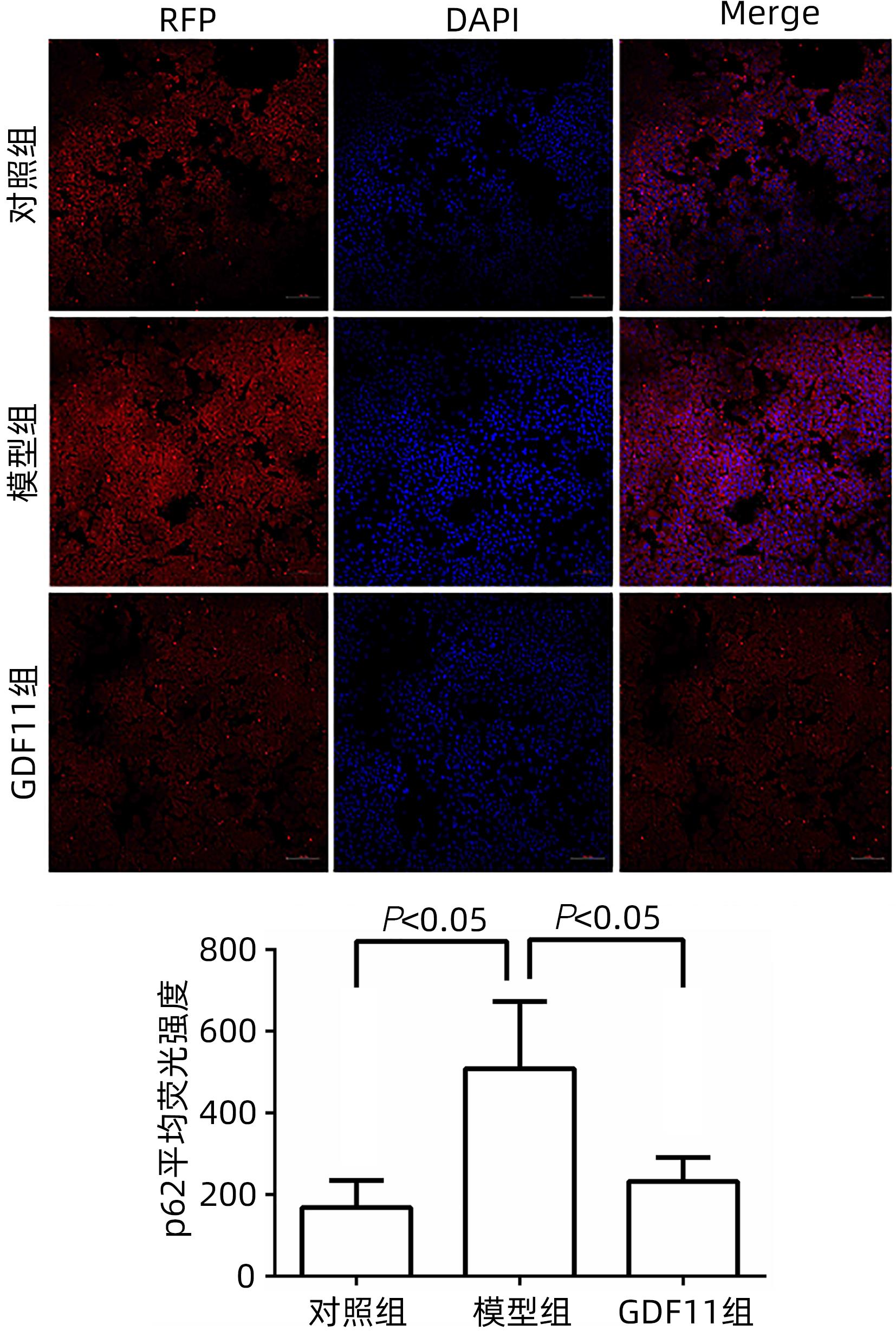
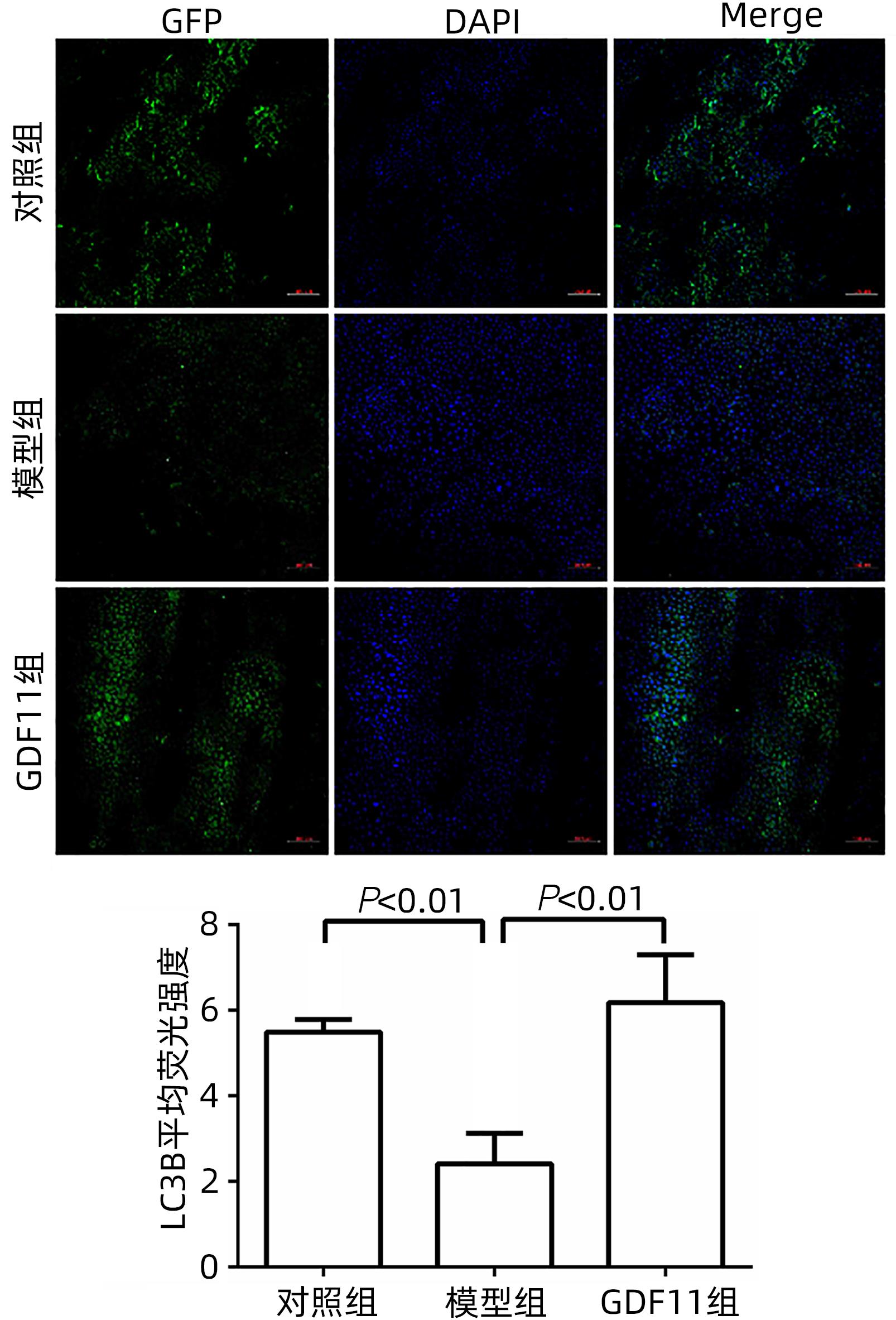
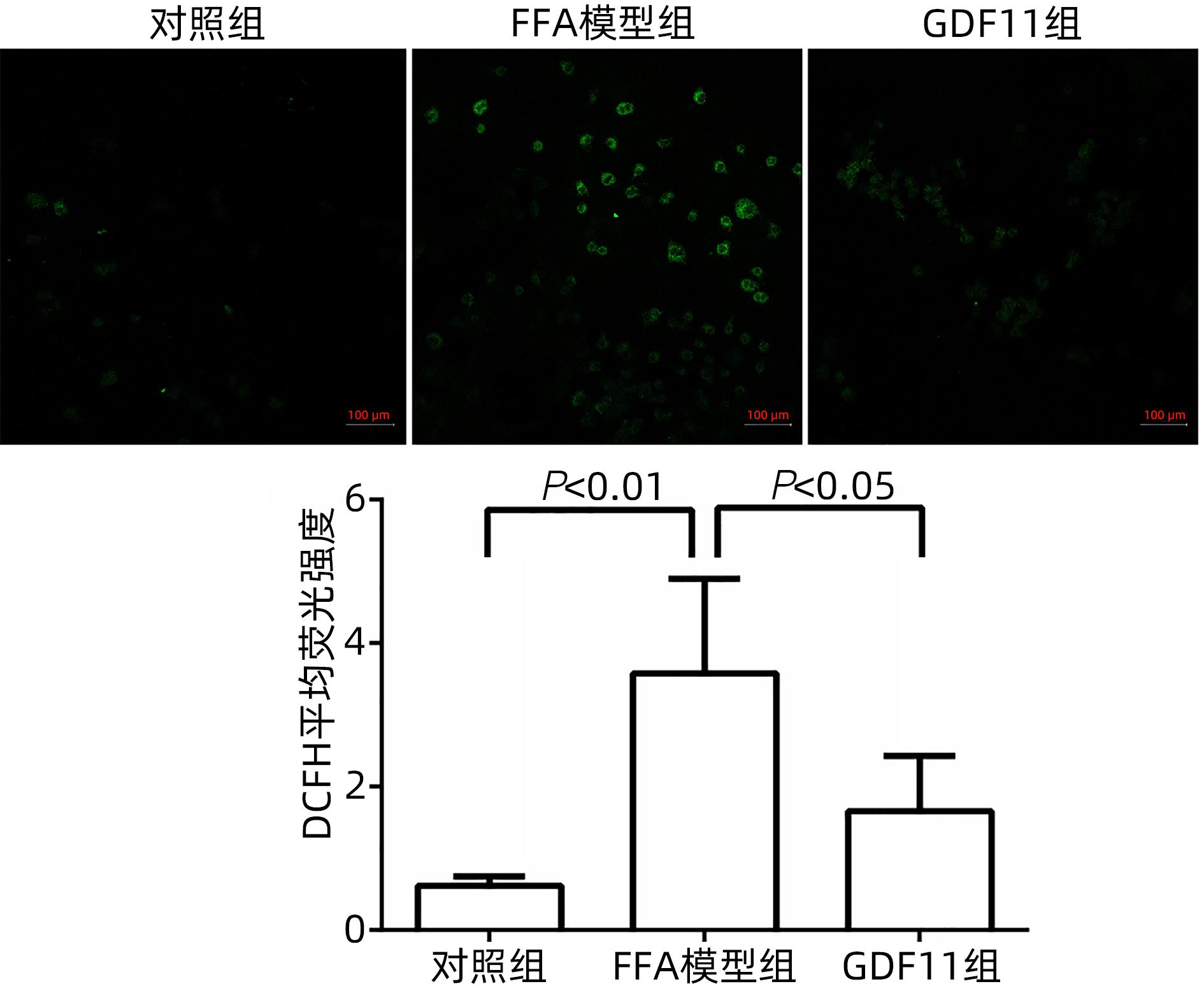
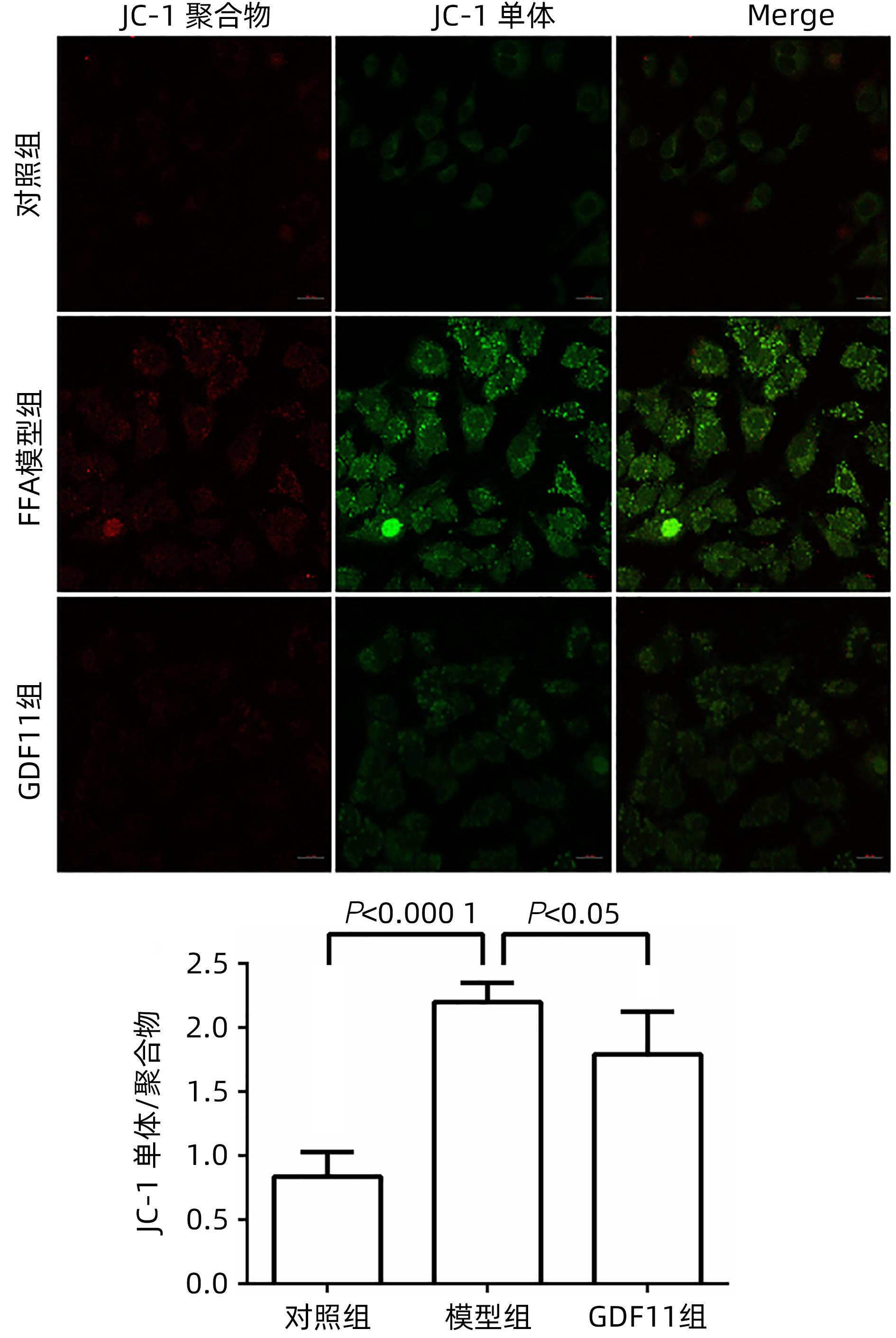
| [1] |
|
| [2] |
RUISSEN MM, MAK AL, BEUERS U, et al. Non-alcoholic fatty liver disease: A multidisciplinary approach towards a cardiometabolic liver disease[J]. Eur J Endocrinol, 2020, 183( 3): R57-R73. DOI: 10.1530/EJE-20-0065. |
| [3] |
TANAKA N, KIMURA T, FUJIMORI N, et al. Current status, problems, and perspectives of non-alcoholic fatty liver disease research[J]. World J Gastroenterol, 2019, 25( 2): 163- 177. DOI: 10.3748/wjg.v25.i2.163. |
| [4] |
LOOMBA R, FRIEDMAN SL, SHULMAN GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease[J]. Cell, 2021, 184( 10): 2537- 2564. DOI: 10.1016/j.cell.2021.04.015. |
| [5] |
GLUCHOWSKI NL, BECUWE M, WALTHER TC, et al. Lipid droplets and liver disease: From basic biology to clinical implications[J]. Nat Rev Gastroenterol Hepatol, 2017, 14( 6): 343- 355. DOI: 10.1038/nrgastro.2017.32. |
| [6] |
KRAHMER N, FARESE RV Jr, WALTHER TC. Balancing the fat: Lipid droplets and human disease[J]. EMBO Mol Med, 2013, 5( 7): 973- 983. DOI: 10.1002/emmm.201100671. |
| [7] |
MORTEZAEE K, KHANLARKHANI N. Melatonin application in targeting oxidative-induced liver injuries: A review[J]. J Cell Physiol, 2018, 233( 5): 4015- 4032. DOI: 10.1002/jcp.26209. |
| [8] |
LINHART KB, GLASSEN K, PECCERELLA T, et al. The generation of carcinogenic etheno-DNA adducts in the liver of patients with nonalcoholic fatty liver disease[J]. Hepatobiliary Surg Nutr, 2015, 4( 2): 117- 123. DOI: 10.3978/j.issn.2304-3881.2015.01.14. |
| [9] |
LIU Q, BENGMARK S, QU S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease(NAFLD)[J]. Lipds Heath Dis, 2010, 9( 1): 1- 9. DOI: 10.1186/1476-511X-9-42. |
| [10] |
WOBSER H, DORN C, WEISS TS, et al. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells[J]. Cell Res, 2009, 19( 8): 996- 1005. DOI: 10.1038/cr.2009.73. |
| [11] |
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68( 6): 394- 424. DOI: 10.3322/caac.21492. |
| [12] |
WALKER RG, POGGIOLI T, KATSIMPARDI L, et al. Biochemistry and biology of GDF11 and myostatin: Similarities, differences, and questions for future investigation[J]. Circ Res, 2016, 118( 7): 1125- 1141. DOI: 10.1161/CIRCRESAHA.116.308391. |
| [13] |
LU BX, ZHONG JN, PAN JF, et al. Gdf11 gene transfer prevents high fat diet-induced obesity and improves metabolic homeostasis in obese and STZ-induced diabetic mice[J]. J Transl Med, 2019, 17( 1): 422. DOI: 10.1186/s12967-019-02166-1. |
| [14] |
FROHLICH J, KOVACOVICOVA K, MAZZA T, et al. GDF11 induces mild hepatic fibrosis independent of metabolic health[J]. Aging, 2020, 12( 20): 20024- 20046. DOI: 10.18632/aging.104182. |
| [15] |
ZHOU J, PANG J, TRIPATHI M, et al. Spermidine-mediated hypusination of translation factor EIF5A improves mitochondrial fatty acid oxidation and prevents non-alcoholic steatohepatitis progression[J]. Nat Commun, 2022, 13( 1): 5202. DOI: 10.1038/s41467-022-32788-x. |
| [16] |
GADIPARTHI C, SPATZ M, GREENBERG S, et al. NAFLD epidemiology, emerging pharmacotherapy, liver transplantation implications and the trends in the United States[J]. J Clin Transl Hepatol, 2020, 8( 2): 215- 221. DOI: 10.14218/JCTH.2020.00014. |
| [17] |
BYRNES K, BLESSINGER S, BAILEY NT, et al. Therapeutic regulation of autophagy in hepatic metabolism[J]. Acta Pharm Sin B, 2022, 12( 1): 33- 49. DOI: 10.1016/j.apsb.2021.07.021. |
| [18] |
ZHONG CC, ZHAO T, HOGSTRAND C, et al. Copper(Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways[J]. J Nutr Biochem, 2022, 100: 108883. DOI: 10.1016/j.jnutbio.2021.108883. |
| [19] |
SCORLETTI E, CARR RM. A new perspective on NAFLD: Focusing on lipid droplets[J]. J Hepatol, 2022, 76( 4): 934- 945. DOI: 10.1016/j.jhep.2021.11.009. |
| [20] |
LIN CW, ZHANG H, LI M, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice[J]. J Hepatol, 2013, 58( 5): 993- 999. DOI: 10.1016/j.jhep.2013.01.011. |
| [21] |
DAI Z, SONG GQ, BALAKRISHNAN A, et al. Growth differentiation factor 11 attenuates liver fibrosis via expansion of liver progenitor cells[J]. Gut, 2020, 69( 6): 1104- 1115. DOI: 10.1136/gutjnl-2019-318812. |
| [22] |
JIAO L, SHAO YC, YU Q, et al. GDF11 replenishment protects against hypoxia-mediated apoptosis in cardiomyocytes by regulating autophagy[J]. Eur J Pharmacol, 2020, 885: 173495. DOI: 10.1016/j.ejphar.2020.173495. |









 本站查看
本站查看
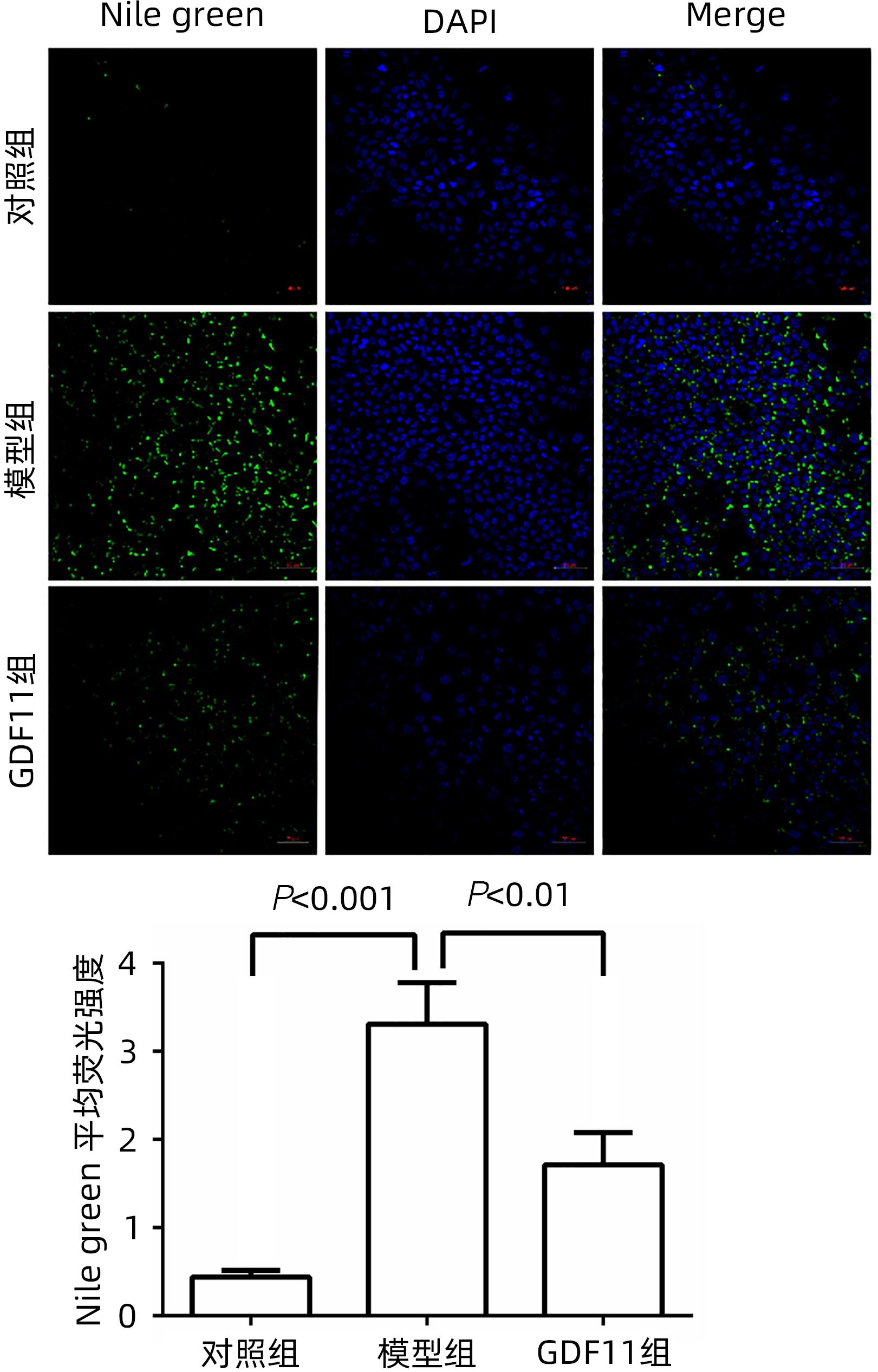




 DownLoad:
DownLoad: