| [1] |
European Association for the Study of the Liver(EASL), European Association for the Study of Diabetes(EASD), European Association for the Study of Obesity(EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease[J]. Obes Facts, 2016, 9( 2): 65- 90. DOI: 10.1159/000443344. |
| [2] |
ZHOU JH, ZHOU F, WANG WX, et al. Epidemiological features of NAFLD from 1999 to 2018 in China[J]. Hepatology, 2020, 71( 5): 1851- 1864. DOI: 10.1002/hep.31150. |
| [3] |
ZHU CX, ZHU Q, WANG C, et al. Hostile takeover: Manipulation of HIF-1 signaling in pathogen-associated cancers(review)[J]. Int J Oncol, 2016, 49( 4): 1269- 1276. DOI: 10.3892/ijo.2016.3633. |
| [4] |
MOYA IM, HALDER G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine[J]. Nat Rev Mol Cell Biol, 2019, 20( 4): 211- 226. DOI: 10.1038/s41580-018-0086-y. |
| [5] |
POLYZOS SA, KOUNTOURAS J, MANTZOROS CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics[J]. Metabolism, 2019, 92: 82- 97. DOI: 10.1016/j.metabol.2018.11.014. |
| [6] |
COMINGUEZ DC, PARK YJ, KANG YM, et al. Clitorin ameliorates western diet-induced hepatic steatosis by regulating lipogenesis and fatty acid oxidation in vivo and in vitro[J]. Sci Rep, 2022, 12( 1): 4154. DOI: 10.1038/s41598-022-07937-3. |
| [7] |
MA B, CHEN Y, CHEN L, et al. Hypoxia regulates hippo signalling through the SIAH2 ubiquitin E3 ligase[J]. Nat Cell Biol, 2015, 17( 1): 95- 103. DOI: 10.1038/ncb3073. |
| [8] |
HE Y, YANG WH, GAN LL, et al. Silencing HIF-1α aggravates non-alcoholic fatty liver disease in vitro through inhibiting PPAR-α/ANGPTL4 singling pathway[J]. Gastroenterol Hepatol, 2021, 44( 5): 355- 365. DOI: 10.1016/j.gastrohep.2020.09.014. |
| [9] |
ARAI T, TANAKA M, GODA N. HIF-1-dependent lipin1 induction prevents excessive lipid accumulation in choline-deficient diet-induced fatty liver[J]. Sci Rep, 2018, 8( 1): 14230. DOI: 10.1038/s41598-018-32586-w. |
| [10] |
KOBAYASHI Y, OGURO A, IMAOKA S. Feedback of hypoxia-inducible factor-1alpha(HIF-1alpha) transcriptional activity via redox factor-1(Ref-1) induction by reactive oxygen species(ROS)[J]. Free Radic Res, 2021, 55( 2): 154- 164. DOI: 10.1080/10715762.2020.1870685. |
| [11] |
JEONG SH, KIM HB, KIM MC, et al. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer[J]. J Clin Invest, 2018, 128( 3): 1010- 1025. DOI: 10.1172/JCI95802. |
| [12] |
SHU ZP, GAO Y, ZHANG GP, et al. A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice[J]. J Cell Mol Med, 2019, 23( 5): 3616- 3628. DOI: 10.1111/jcmm.14262. |
| [13] |
HU Y, SHIN DJ, PAN H, et al. YAP suppresses gluconeogenic gene expression through PGC1α[J]. Hepatology, 2017, 66( 6): 2029- 2041. DOI: 10.1002/hep.29373. |
| [14] |
WONG RJ, CHEUNG R, AHMED A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S[J]. Hepatology, 2014, 59( 6): 2188- 2195. DOI: 10.1002/hep.26986. |
| [15] |
CAI N, ZHAO X, JING YY, et al. Autophagy protects against palmitate-induced apoptosis in hepatocytes[J]. Cell Biosci, 2014, 4: 28. DOI: 10.1186/2045-3701-4-28. |
| [16] |
WANG XJ, de CARVALHO RIBEIRO M, IRACHETA-VELLVE A, et al. Macrophage-specific hypoxia-inducible factor-1α contributes to impaired autophagic flux in nonalcoholic steatohepatitis[J]. Hepatology, 2019, 69( 2): 545- 563. DOI: 10.1002/hep.30215. |
| [17] |
HERNÁNDEZ A, GENG YN, SEPÚLVEDA R, et al. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles[J]. Biochim Biophys Acta Mol Basis Dis, 2020, 1866( 6): 165753. DOI: 10.1016/j.bbadis.2020.165753. |
| [18] |
LI T, XIAN WJ, GAO Y, et al. Higd1a protects cells from lipotoxicity under high-fat exposure[J]. Oxid Med Cell Longev, 2019, 2019: 6051262. DOI: 10.1155/2019/6051262. |
| [19] |
SHIN MK, DRAGER LF, YAO QL, et al. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α[J]. PLoS One, 2012, 7( 10): e46562. DOI: 10.1371/journal.pone.0046562. |
| [20] |
LUEDDE T, KAPLOWITZ N, SCHWABE RF. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance[J]. Gastroenterology, 2014, 147( 4): 765- 783. DOI: 10.1053/j.gastro.2014.07.018. |
| [21] |
PIERANTONELLI I, SVEGLIATI-BARONI G. Nonalcoholic fatty liver disease: Basic pathogenetic mechanisms in the progression from NAFLD to NASH[J]. Transplantation, 2019, 103( 1): e1-e13. DOI: 10.1097/TP.0000000000002480. |
| [22] |
WREE A, BRODERICK L, CANBAY A, et al. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms[J]. Nat Rev Gastroenterol Hepatol, 2013, 10( 11): 627- 636. DOI: 10.1038/nrgastro.2013.149. |
| [23] |
YOO W, NOH KH, AHN JH, et al. HIF-1α expression as a protective strategy of HepG2 cells against fatty acid-induced toxicity[J]. J Cell Biochem, 2014, 115( 6): 1147- 1158. DOI: 10.1002/jcb.24757. |
| [24] |
CHA JY, KIM DH, CHUN KH. The role of hepatic macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Lab Anim Res, 2018, 34( 4): 133- 139. DOI: 10.5625/lar.2018.34.4.133. |
| [25] |
TOSELLO-TRAMPONT AC, LANDES SG, NGUYEN V, et al. Kupffer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production[J]. J Biol Chem, 2012, 287( 48): 40161- 40172. DOI: 10.1074/jbc.M112.417014. |
| [26] |
ARDESTANI A, LUPSE B, MAEDLER K. Hippo signaling: Key emerging pathway in cellular and whole-body metabolism[J]. Trends Endocrinol Metab, 2018, 29( 7): 492- 509. DOI: 10.1016/j.tem.2018.04.006. |
| [27] |
SONG K, KWON H, HAN C, et al. Yes-associated protein in kupffer cells enhances the production of proinflammatory cytokines and promotes the development of nonalcoholic steatohepatitis[J]. Hepatology, 2020, 72( 1): 72- 87. DOI: 10.1002/hep.30990. |
| [28] |
YIMLAMAI D, CHRISTODOULOU C, GALLI GG, et al. Hippo pathway activity influences liver cell fate[J]. Cell, 2014, 157( 6): 1324- 1338. DOI: 10.1016/j.cell.2014.03.060. |
| [29] |
MOORING M, FOWL BH, LUM SZC, et al. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis[J]. Hepatology, 2020, 71( 5): 1813- 1830. DOI: 10.1002/hep.30928. |
| [30] |
CHEN P, LUO QH, HUANG C, et al. Pathogenesis of non-alcoholic fatty liver disease mediated by YAP[J]. Hepatol Int, 2018, 12( 1): 26- 36. DOI: 10.1007/s12072-017-9841-y. |
| [31] |
WU W, LI WP, WEI JJ, et al. Chronic intermittent hypoxia accelerates liver fibrosis in rats with combined hypoxia and nonalcoholic steatohepatitis via angiogenesis rather than endoplasmic reticulum stress[J]. Acta Biochim Biophys Sin, 2019, 51( 2): 159- 167. DOI: 10.1093/abbs/gmy169. |
| [32] |
HAN J, HE YP, ZHAO H, et al. Hypoxia inducible factor-1 promotes liver fibrosis in nonalcoholic fatty liver disease by activating PTEN/p65 signaling pathway[J]. J Cell Biochem, 2019, 120( 9): 14735- 14744. DOI: 10.1002/jcb.28734. |
| [33] |
MESARWI OA, SHIN MK, BEVANS-FONTI S, et al. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease[J]. PLoS One, 2016, 11( 12): e0168572. DOI: 10.1371/journal.pone.0168572. |
| [34] |
ZHAN L, HUANG C, MENG XM, et al. Hypoxia-inducible factor-1alpha in hepatic fibrosis: A promising therapeutic target[J]. Biochimie, 2015, 108: 1- 7. DOI: 10.1016/j.biochi.2014.10.013. |
| [35] |
KAMINSKY-KOLESNIKOV Y, RAUCHBACH E, ABU-HALAKA D, et al. Cholesterol induces nrf-2- and HIF-1α-dependent hepatocyte proliferation and liver regeneration to ameliorate bile acid toxicity in mouse models of NASH and fibrosis[J]. Oxid Med Cell Longev, 2020, 2020: 5393761. DOI: 10.1155/2020/5393761. |
| [36] |
SALLOUM S, JEYARAJAN AJ, KRUGER AJ, et al. Fatty acids activate the transcriptional coactivator YAP1 to promote liver fibrosis via p38 mitogen-activated protein kinase[J]. Cell Mol Gastroenterol Hepatol, 2021, 12( 4): 1297- 1310. DOI: 10.1016/j.jcmgh.2021.06.003. |
| [37] |
SONG ZL, CHEN W, ATHAVALE D, et al. Osteopontin takes center stage in chronic liver disease[J]. Hepatology, 2021, 73( 4): 1594- 1608. DOI: 10.1002/hep.31582. |
| [38] |
MACHADO MV, MICHELOTTI GA, PEREIRA TA, et al. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease[J]. J Hepatol, 2015, 63( 4): 962- 970. DOI: 10.1016/j.jhep.2015.05.031. |
| [39] |
MARTIN K, PRITCHETT J, LLEWELLYN J, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis[J]. Nat Commun, 2016, 7: 12502. DOI: 10.1038/ncomms12502. |
| [40] |
SHEKA AC, ADEYI O, THOMPSON J, et al. Nonalcoholic steatohepatitis: A review[J]. JAMA, 2020, 323( 12): 1175- 1183. DOI: 10.1001/jama.2020.2298. |
| [41] |
LI JP, SUN Y, KONG XB, et al. Changes of serum cytokeratin-18 fragment M30,adiponectin and retinol binding protein 4 in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease and their clinical significance[J]. J Clin Exp Med, 2022, 21( 9): 941- 945. DOI: 10.3969/j.issn.1671-4695.2022.09.012. |
| [42] |
WONG VW, WONG GL, WOO J, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease[J]. Clin Gastroenterol Hepatol, 2021, 19( 10): 2161- 2171. DOI: 10.1016/j.cgh.2020.10.046. |
| [43] |
GONZALEZ FJ, XIE C, JIANG CT. The role of hypoxia-inducible factors in metabolic diseases[J]. Nat Rev Endocrinol, 2018, 15( 1): 21- 32. DOI: 10.1038/s41574-018-0096-z. |
| [44] |
ANENI EC, ONI ET, MARTIN SS, et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk[J]. J Hypertens, 2015, 33( 6): 1207- 1214. DOI: 10.1097/HJH.0000000000000532. |
| [45] |
BONNET F, GASTALDELLI A, PIHAN-LE BARS F, et al. Gamma-glutamyltransferase, fatty liver index and hepatic insulin resistance are associated with incident hypertension in two longitudinal studies[J]. J Hypertens, 2017, 35( 3): 493- 500. DOI: 10.1097/HJH.0000000000001204. |
| [46] |
BURGUEÑO AL, GIANOTTI TF, MANSILLA NG, et al. Cardiovascular disease is associated with high-fat-diet-induced liver damage and up-regulation of the hepatic expression of hypoxia-inducible factor 1α in a rat model[J]. Clin Sci, 2013, 124( 1): 53- 63. DOI: 10.1042/CS20120151. |
| [47] |
ZHANG XM, LAM KSL, YE HY, et al. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha}induces obesity and glucose intolerance by impeding energy expenditure in mice[J]. J Biol Chem, 2010, 285( 43): 32869- 32877. DOI: 10.1074/jbc.M110.135509. |
| [48] |
ASAI Y, YAMADA T, TSUKITA S, et al. Activation of the hypoxia inducible factor 1α subunit pathway in steatotic liver contributes to formation of cholesterol gallstones[J]. Gastroenterology, 2017, 152( 6): 1521- 1535. DOI: 10.1053/j.gastro.2017.01.001. |



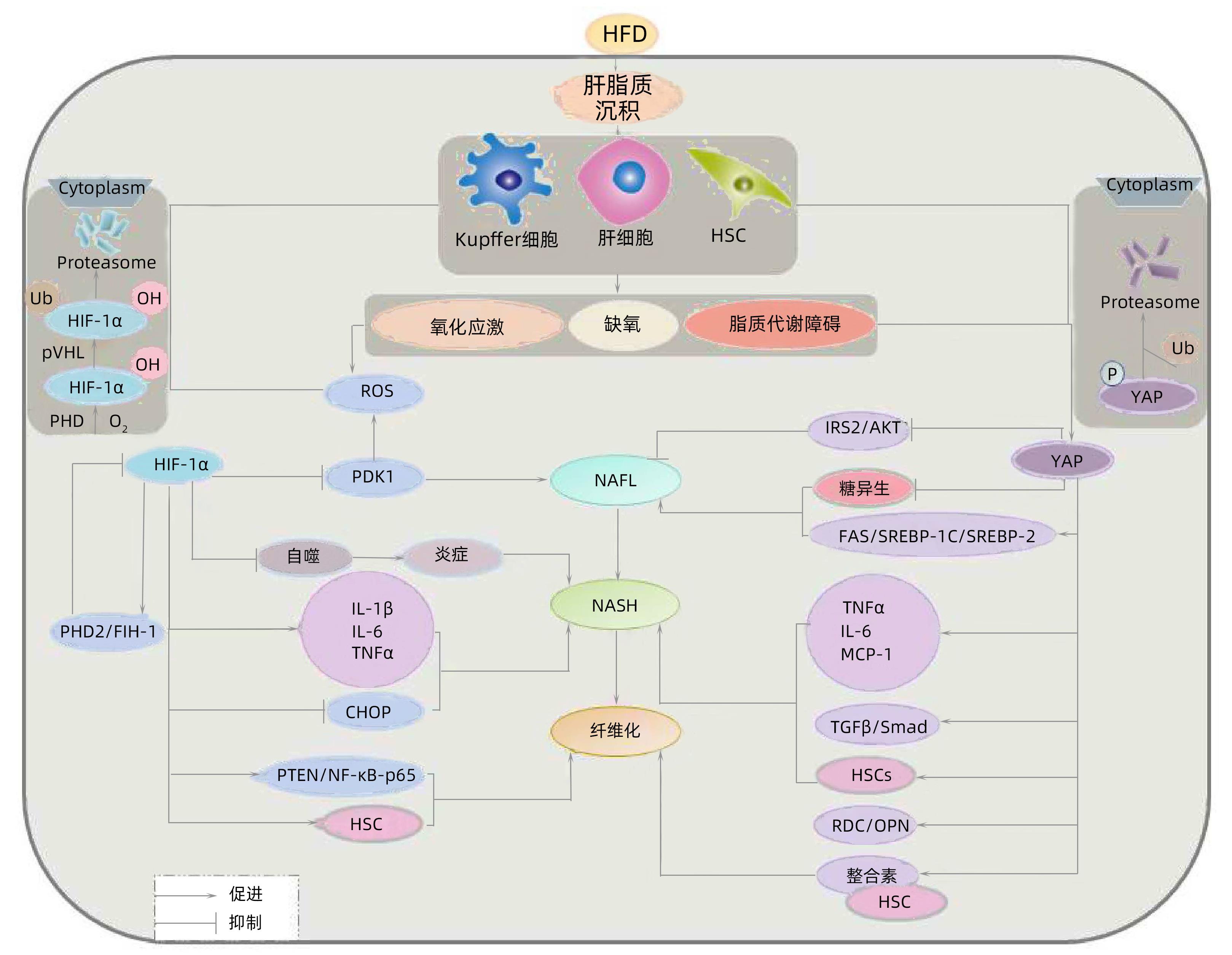




 DownLoad:
DownLoad: