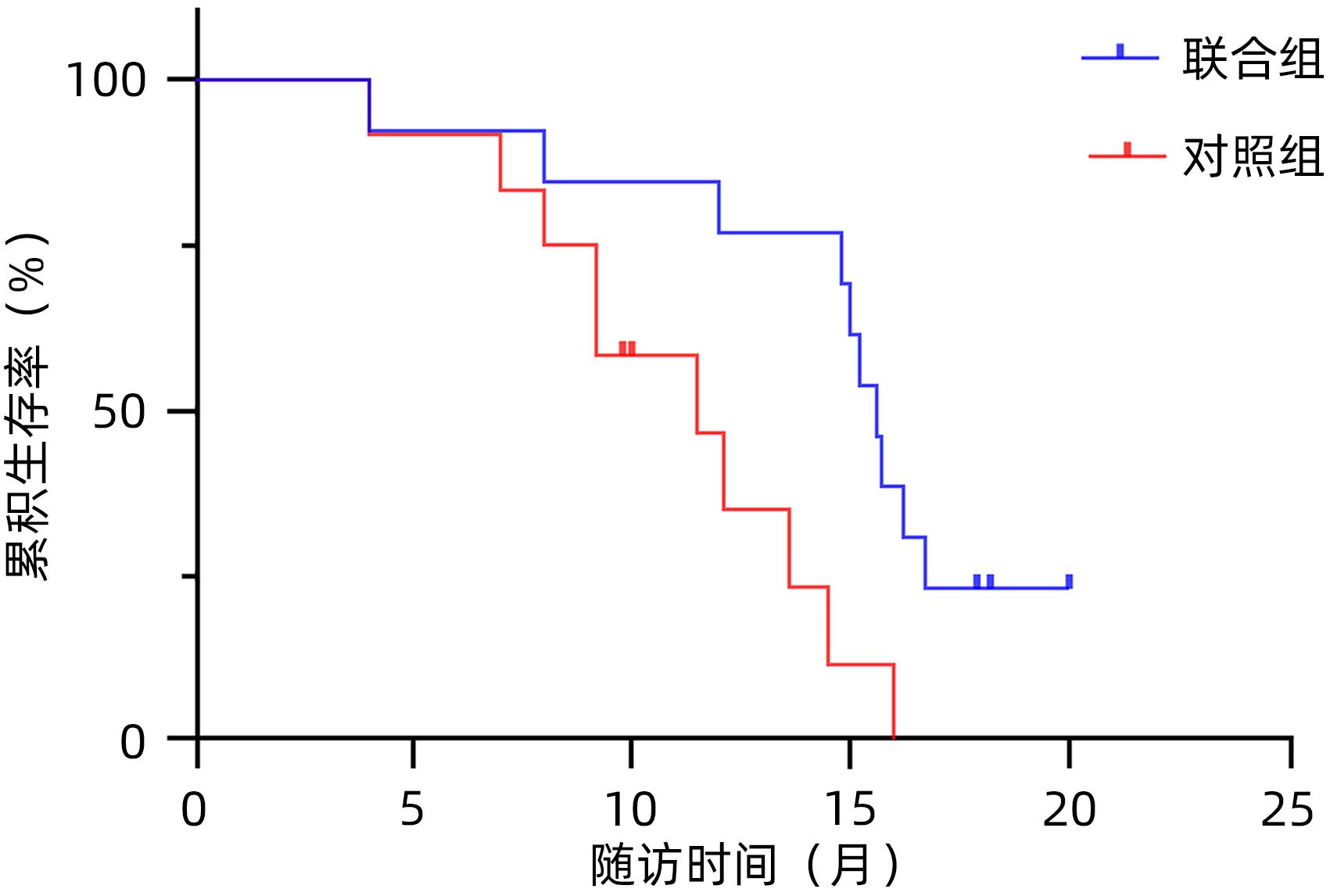
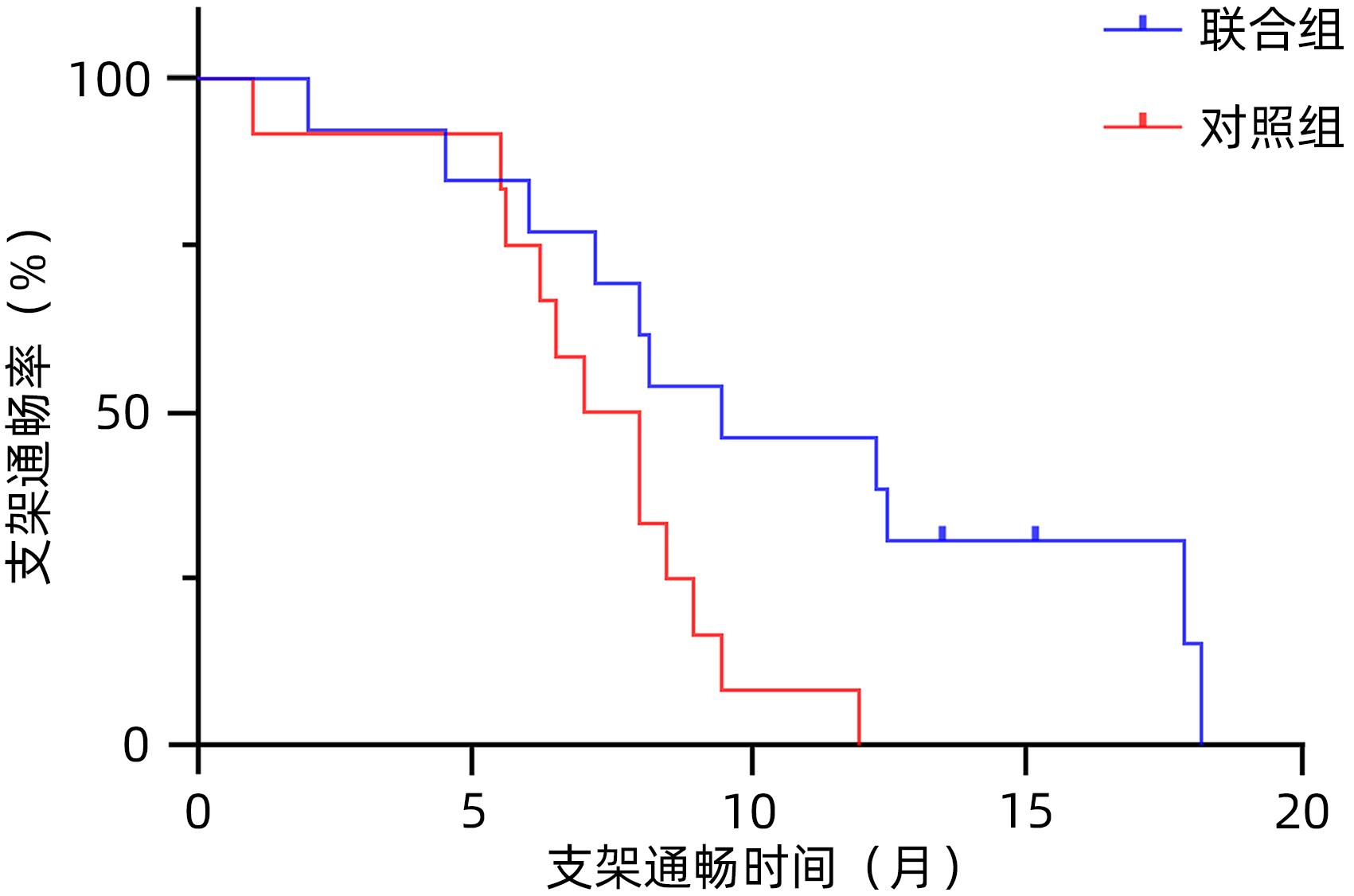
| [1] |
RIZVI S, KHAN SA, HALLEMEIER CL, et al. Cholangiocarcinoma-evolving concepts and therapeutic strategies[J]. Nat Rev Clin Oncol, 2018, 15( 2): 95- 111. DOI: 10.1038/nrclinonc.2017.157. |
| [2] |
FORNER A, VIDILI G, RENGO M, et al. Clinical presentation, diagnosis and staging of cholangiocarcinoma[J]. Liver Int, 2019, 39( Suppl 1): 98- 107. DOI: 10.1111/liv.14086. |
| [3] |
VALLE J, WASAN H, PALMER DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362( 14): 1273- 1281. DOI: 10.1056/NEJMoa0908721. |
| [4] |
JIANG X, YANG JJ, LI H. Clinical effect of brachytherapy with 125I seed implantation in treatment of malignant hepatopancreatobiliary tumors[J]. J Clin Hepatol, 2016, 32( 12): 2300- 2304. DOI: 10.3969/j.issn.1001-5256.2016.12.014. |
| [5] |
JIAO D, ZHOU X, LI Z, et al. A newly designed biliary brachytherapy drainage catheter for patients with malignant biliary obstruction: A pilot study[J]. J Cancer Res Ther, 2020, 16( 2): 286- 291. DOI: 10.4103/jcrt.JCRT_804_19. |
| [6] |
PAN T, LI MA, MU LW, et al. Stent placement with iodine-125 seeds strand effectively extends the duration of stent patency and survival in patients with unresectable malignant obstructive jaundice[J]. Scand J Gastroenterol, 2020, 55( 1): 123- 128. DOI: 10.1080/00365521.2019.1707275. |
| [7] |
UENO M, IKEDA M, SASAKI T, et al. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: primary analysis results[J]. BMC Cancer, 2020, 20( 1): 1105. DOI: 10.1186/s12885-020-07365-4. |
| [8] |
WANG Y, YANG X, WANG D, et al. Lenvatinib beyond first-line therapy in patients with advanced biliary tract carcinoma[J]. Front Oncol, 2022, 12: 785535. DOI: 10.3389/fonc.2022.785535. |
| [9] |
HAN XW, LI YD, MA B, et al. Percutaneous transhepatic cholangiographic forceps biopsy in pathologic diagnosis for obstructive jaundice[J]. Chin J Radiol, 2004, 38( 10): 1025- 1029. DOI: 10.3760/j.issn:1005-1201.2004.10.003. |
| [10] |
CHAI J, LU D, LYU WF, et al. Percutaneous transhepatic biliary 125I seeds stent implantation for malignant biliary obstruction:analysis of its efficacy and safety[J]. J Intervent Radiol, 2022, 31( 7): 702- 706. DOI: 10.3969/j.issn.1008-794X.2022.07.014. |
| [11] |
WANG XJ, LI WH, HUANG R. Difference of curative effect between single biliary stent and biliary stent combined with 125I seed in the treatment of malignant obstructive jaundice[J]. J Pract Radiol, 2019, 35( 9): 1488- 1492. DOI: 10.3969/j.issn.1002-1671.2019.09.027. |
| [12] |
HU XS, PANG Q, LIU HC, et al. Percutaneous biliary metallic stent implantation combined with catheter-loaded 125I seeds in the treatment of locally advanced extrahepatic cholangiocarcinoma: evaluation of curative effect and analysis of prognostic factors[J]. J Intervent Radiol, 2019, 28( 4): 369- 375. DOI: 10.3969/j.issn.1008-794X.2019.04.015. |
| [13] |
|
| [14] |
EL-KHOUEIRY AB, RANKIN CJ, BEN-JOSEF E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma[J]. Invest New Drugs, 2012, 30( 4): 1646- 1651. DOI: 10.1007/s10637-011-9719-0. |
| [15] |
SUN W, PATEL A, NORMOLLE D, et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma[J]. Cancer, 2019, 125( 6): 902- 909. DOI: 10.1002/cncr.31872. |
| [16] |
XU YF, YANG XQ, LU XF, et al. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma[J]. Biochem Biophys Res Commun, 2014, 446( 1): 54- 60. DOI: 10.1016/j.bbrc.2014.02.050. |
| [17] |
BOONJARASPINYO S, BOONMARS T, WU Z, et al. Platelet-derived growth factor may be a potential diagnostic and prognostic marker for cholangiocarcinoma[J]. Tumour Biol, 2012, 33( 5): 1785- 1802. DOI: 10.1007/s13277-012-0438-8. |
| [18] |
YOSHIKAWA D, OJIMA H, IWASAKI M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma[J]. Br J Cancer, 2008, 98( 2): 418- 425. DOI: 10.1038/sj.bjc.6604129. |
| [19] |
OKAMOTO K, KODAMA K, TAKASE K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib(E7080) against RET gene fusion-driven tumor models[J]. Cancer Lett, 2013, 340( 1): 97- 103. DOI: 10.1016/j.canlet.2013.07.007. |
| [20] |
MATSUI J, YAMAMOTO Y, FUNAHASHI Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition[J]. Int J Cancer, 2008, 122( 3): 664- 671. DOI: 10.1002/ijc.23131. |
| [21] |
YAMAMOTO Y, MATSUI J, MATSUSHIMA T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage[J]. Vasc Cell, 2014, 6: 18. DOI: 10.1186/2045-824X-6-18. |
| [22] |
TOHYAMA O, MATSUI J, KODAMA K, et al. Antitumor activity of lenvatinib(e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models[J]. J Thyroid Res, 2014, 2014: 638747. DOI: 10.1155/2014/638747. |
| [23] |
TORRENS L, MONTIRONI C, PUIGVEHÍ M, et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma[J]. Hepatology, 2021, 74( 5): 2652- 2669. DOI: 10.1002/hep.32023. |



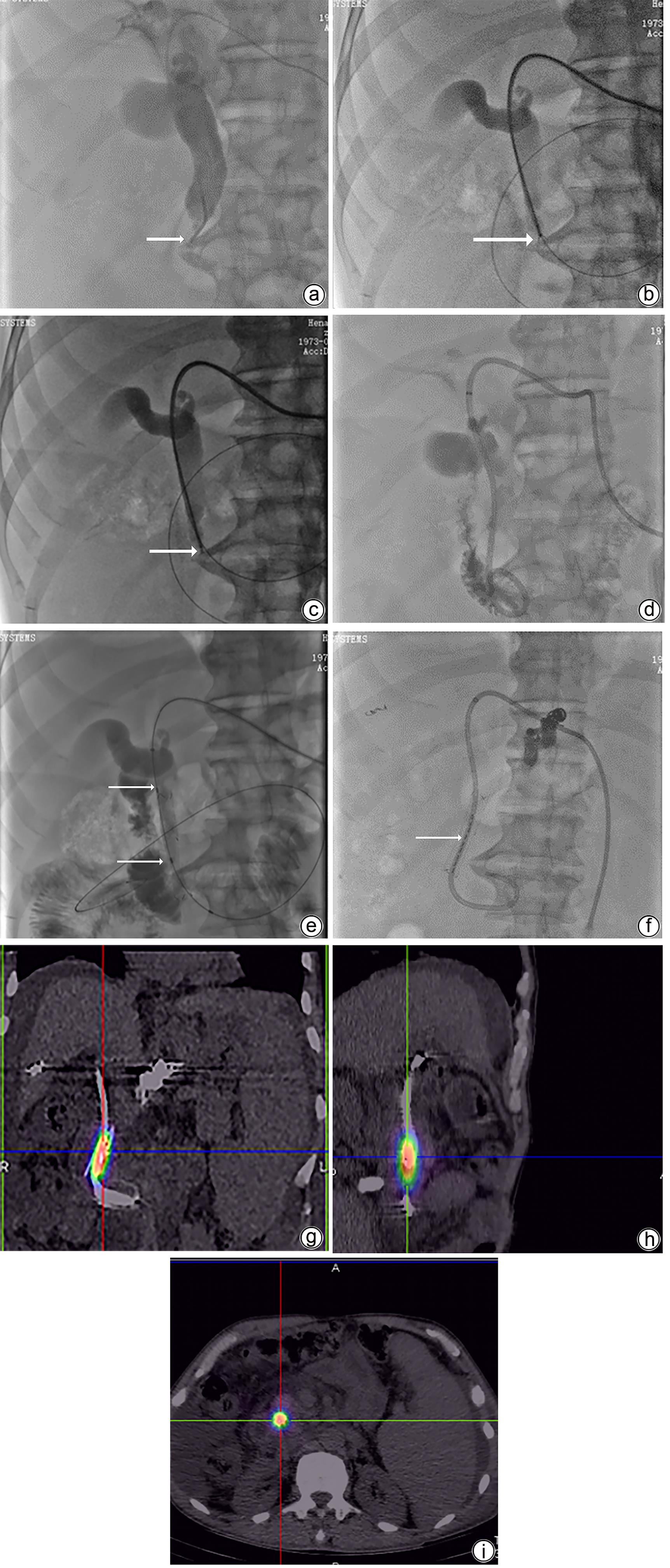




 DownLoad:
DownLoad: