| [1] |
CHEN J, HUANG AL. Functional cure of hepatitis B from the perspective of hepatitis B virus covalently closed circular DNA[J]. J Clin Hepatol, 2022, 38( 8): 1716- 1720. DOI: 10.3969/j.issn.1001-5256.2022.08.003. |
| [2] |
BUCHNER J. Molecular chaperones and protein quality control: an introduction to the JBC Reviews thematic series[J]. J Biol Chem, 2019, 294( 6): 2074- 2075. DOI: 10.1074/jbc.REV118.006739. |
| [3] |
HU C, YANG J, QI Z, et al. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities[J]. MedComm(2020), 2022, 3( 3): e161. DOI: 10.1002/mco2.161. |
| [4] |
LANG BJ, PRINCE TL, OKUSHA Y, et al. Heat shock proteins in cell signaling and cancer[J]. Biochim Biophys Acta Mol Cell Res, 2022, 1869( 3): 119187. DOI: 10.1016/j.bbamcr.2021.119187. |
| [5] |
JAKOB U, LILIE H, MEYER I, et al. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo[J]. J Biol Chem, 1995, 270( 13): 7288- 7294. DOI: 10.1074/jbc.270.13.7288. |
| [6] |
WIECH H, BUCHNER J, ZIMMERMANN R, et al. Hsp90 chaperones protein folding in vitro[J]. Nature, 1992, 358( 6382): 169- 170. DOI: 10.1038/358169a0. |
| [7] |
CASTRO JP, FERNANDO R, REEG S, et al. Non-enzymatic cleavage of Hsp90 by oxidative stress leads to actin aggregate formation: A novel gain-of-function mechanism[J]. Redox Biol, 2019, 21: 101108. DOI: 10.1016/j.redox.2019.101108. |
| [8] |
KIM MJ, KIM J, IM JS, et al. Hepatitis B virus X protein enhances liver cancer cell migration by regulating calmodulin-associated actin polymerization[J]. BMB Rep, 2021, 54( 12): 614- 619. DOI: 10.5483/BMBRep.2021.54.12.084. |
| [9] |
WEIS F, MOULLINTRAFFORT L, HEICHETTE C, et al. The 90-kDa heat shock protein Hsp90 protects tubulin against thermal denaturation[J]. J Biol Chem, 2010, 285( 13): 9525- 9534. DOI: 10.1074/jbc.M109.096586. |
| [10] |
KRTKOVÁ J, ZIMMERMANN A, SCHWARZEROVÁ K, et al. Hsp90 binds microtubules and is involved in the reorganization of the microtubular network in angiosperms[J]. J Plant Physiol, 2012, 169( 14): 1329- 1339. DOI: 10.1016/j.jplph.2012.06.010. |
| [11] |
HALLETT ST, PASTOK MW, MORGAN R, et al. Differential regulation of G1 CDK complexes by the Hsp90-Cdc37 chaperone system[J]. Cell Rep, 2017, 21( 5): 1386- 1398. DOI: 10.1016/j.celrep.2017.10.042. |
| [12] |
MIKOLAJCZYK M, NELSON MA. Regulation of stability of cyclin-dependent kinase CDK11 p110 and a caspase-processed form, CDK11 p46, by Hsp90[J]. Biochem J, 2004, 384( Pt 3): 461- 467. DOI: 10.1042/BJ20040848. |
| [13] |
ALIGUE R, AKHAVAN-NIAK H, RUSSELL P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90[J]. EMBO J, 1994, 13( 24): 6099- 6106. DOI: 10.1002/j.1460-2075.1994.tb06956.x. |
| [14] |
MEGAHED F, ZHOU X, SUN P. The interactions between HBV and the innate immunity of hepatocytes[J]. Viruses, 2020, 12( 3): 285. DOI: 10.3390/v12030285. |
| [15] |
|
| [16] |
SHIM HY, QUAN X, YI YS, et al. Heat shock protein 90 facilitates formation of the HBV capsid via interacting with the HBV core protein dimers[J]. Virology, 2011, 410( 1): 161- 169. DOI: 10.1016/j.virol.2010.11.005. |
| [17] |
TAVERNITI V, LIGAT G, DEBING Y, et al. Capsid assembly modulators as antiviral agents against HBV: Molecular mechanisms and clinical perspectives[J]. J Clin Med, 2022, 11( 5): 1349. DOI: 10.3390/jcm11051349. |
| [18] |
ZHAO Q, HU Z, CHENG J, et al. Hepatitis B virus core protein dephosphorylation occurs during pregenomic RNA encapsidation[J]. J Virol, 2018, 92( 13): e02139- 17. DOI: 10.1128/JVI.02139-17. |
| [19] |
MAKOKHA GN, ABE-CHAYAMA H, CHOWDHURY S, et al. Regulation of the Hepatitis B virus replication and gene expression by the multi-functional protein TARDBP[J]. Sci Rep, 2019, 9( 1): 8462. DOI: 10.1038/s41598-019-44934-5. |
| [20] |
ZOULIM F, SEEGER C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase[J]. J Virol, 1994, 68( 1): 6- 13. DOI: 10.1128/JVI.68.1.6-13.1994. |
| [21] |
LIU XQ, OHSAKI E, UEDA K. Establishment of a system for finding inhibitors of ε RNA binding with the HBV polymerase[J]. Genes Cells, 2020, 25( 8): 523- 537. DOI: 10.1111/gtc.12778. |
| [22] |
HU J, FLORES D, TOFT D, et al. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function[J]. J Virol, 2004, 78( 23): 13122- 13131. DOI: 10.1128/JVI.78.23.13122-13131.2004. |
| [23] |
TAJWAR R, BRADLEY DP, PONZAR NL, et al. Predicted structure of the hepatitis B virus polymerase reveals an ancient conserved protein fold[J]. Protein Sci, 2022, 31( 10): e4421. DOI: 10.1002/pro.4421. |
| [24] |
GYOO PARK S, KYUNG RHO J, JUNG G. Hsp90 makes the human HBV Pol competent for in vitro priming rather than maintaining the human HBV Pol/pregenomic RNA complex[J]. Arch Biochem Biophys, 2002, 401( 1): 99- 107. DOI: 10.1016/S0003-9861(02)00004-8. |
| [25] |
KIM YS, SEO HW, JUNG G. Reactive oxygen species promote heat shock protein 90-mediated HBV capsid assembly[J]. Biochem Biophys Res Commun, 2015, 457( 3): 328- 333. DOI: 10.1016/j.bbrc.2014.12.110. |
| [26] |
LIM SO, PARK SG, YOO JH, et al. Expression of heat shock proteins(HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules[J]. World J Gastroenterol, 2005, 11( 14): 2072- 2079. DOI: 10.3748/wjg.v11.i14.2072. |
| [27] |
SEO HW, SEO JP, JUNG G. Heat shock protein 70 and heat shock protein 90 synergistically increase hepatitis B viral capsid assembly[J]. Biochem Biophys Res Commun, 2018, 503( 4): 2892- 2898. DOI: 10.1016/j.bbrc.2018.08.065. |
| [28] |
|
| [29] |
TURELLI P, MANGEAT B, JOST S, et al. Inhibition of hepatitis B virus replication by APOBEC3G[J]. Science, 2004, 303( 5665): 1829. DOI: 10.1126/science.1092066. |
| [30] |
NGUYEN DH, HU J. Reverse transcriptase- and RNA packaging signal-dependent incorporation of APOBEC3G into hepatitis B virus nucleocapsids[J]. J Virol, 2008, 82( 14): 6852- 6861. DOI: 10.1128/JVI.00465-08. |
| [31] |
CHEN Z, EGGERMAN TL, BOCHAROV AV, et al. Heat shock proteins stimulate APOBEC-3-mediated cytidine deamination in the hepatitis B virus[J]. J Biol Chem, 2017, 292( 32): 13459- 13479. DOI: 10.1074/jbc.M116.760637. |
| [32] |
MITRA B, THAPA RJ, GUO H, et al. Host functions used by hepatitis B virus to complete its life cycle: Implications for developing host-targeting agents to treat chronic hepatitis B[J]. Antiviral Res, 2018, 158: 185- 198. DOI: 10.1016/j.antiviral.2018.08.014. |
| [33] |
BROUGH PA, AHERNE W, BARRIL X, et al. 4,5-diarylisoxazole Hsp90 chaperone inhibitors: potential therapeutic agents for the treatment of cancer[J]. J Med Chem, 2008, 51( 2): 196- 218. DOI: 10.1021/jm701018h. |
| [34] |
HU KH, HUANG YY, MU JF, et al. KNK437 inhibits replication and transcription of the hepatitis B virus[J]. Chin J Virol, 2017, 33( 1): 24- 35. DOI: 10.13242/j.cnki.bingduxuebao.003090. |
| [35] |
HU L, WANG Y, CHEN Z, et al. Hsp90 inhibitor SNX-2112 enhances TRAIL-induced apoptosis of human cervical cancer cells via the ROS-mediated JNK-p53-autophagy-DR5 pathway[J]. Oxid Med Cell Longev, 2019, 2019: 9675450. DOI: 10.1155/2019/9675450. |
| [36] |
GUAN L, ZOU Q, LIU Q, et al. HSP90 inhibitor ganetespib(STA-9090) inhibits tumor growth in c-Myc-dependent esophageal squamous cell carcinoma[J]. Onco Targets Ther, 2020, 13: 2997- 3011. DOI: 10.2147/OTT.S245813. |



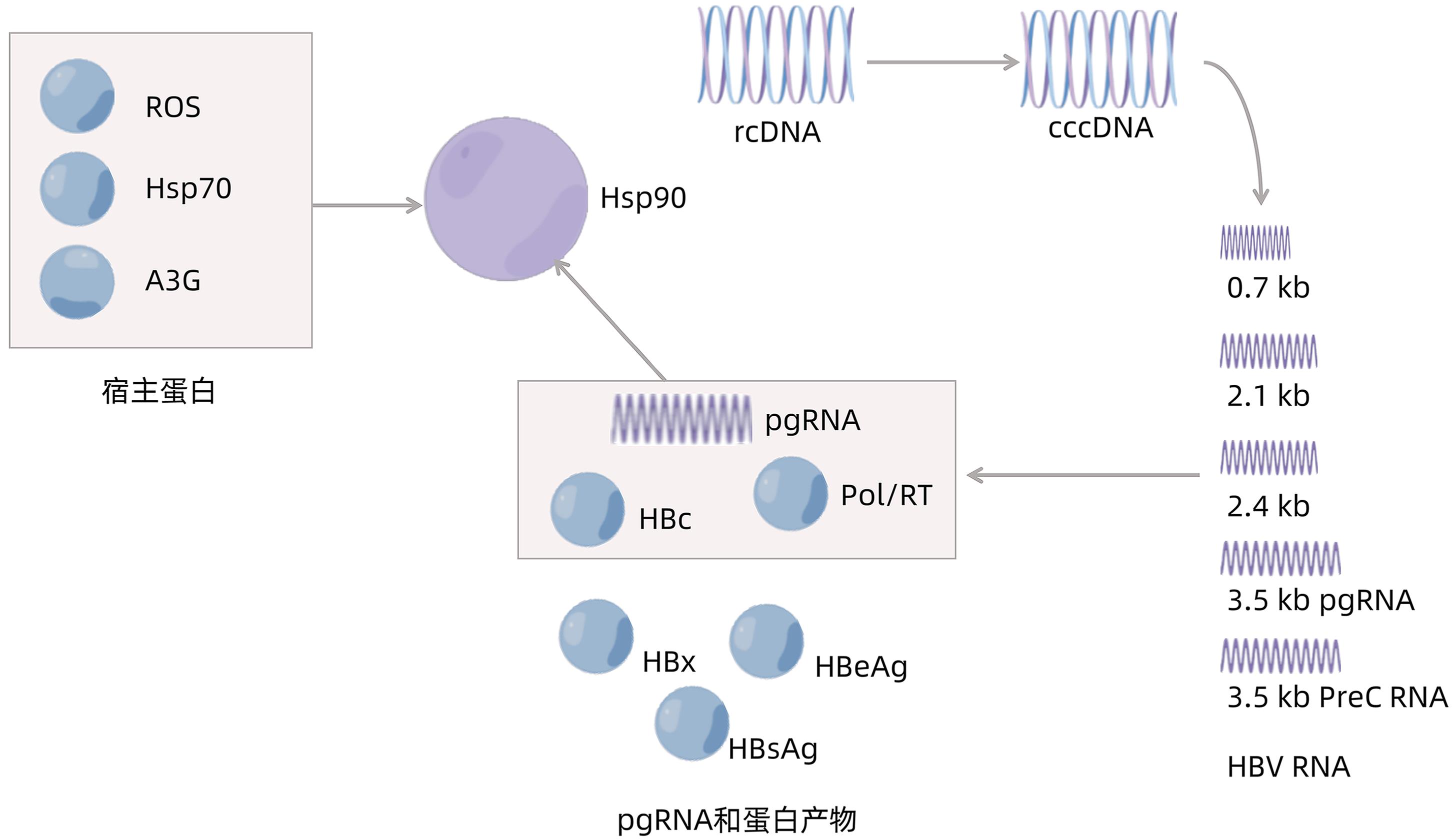




 DownLoad:
DownLoad: