| [1] |
SMITH DB, IZOPET J, NICOT F, et al. Update: proposed reference sequences for subtypes of hepatitis E virus(species Orthohepevirus A)[J]. J Gen Virol, 2020, 101( 7): 692- 698. DOI: 10.1099/jgv.0.001435. |
| [2] |
LHOMME S, LEGRAND-ABRAVANEL F, KAMAR N, et al. Screening, diagnosis and risks associated with hepatitis E virus infection[J]. Expert Rev Anti Infect Ther, 2019, 17( 6): 403- 418. DOI: 10.1080/14787210.2019.1613889. |
| [3] |
BES M, COSTAFREDA MI, RIVEIRO-BARCIELA M, et al. Effect of hepatitis E virus RNA universal blood donor screening, Catalonia, Spain, 2017-2020[J]. Emerg Infect Dis, 2022, 28( 1): 157- 165. DOI: 10.3201/eid2801.211466. |
| [4] |
CHILAKA VN, KONJE JC. Viral hepatitis in pregnancy[J]. Eur J Obstet Gynecol Reprod Biol, 2021, 256: 287- 296. DOI: 10.1016/j.ejogrb.2020.11.052. |
| [5] |
QASHQARI FS. Seroprevalence of hepatitis E virus infection in Middle Eastern Countries: A systematic review and Meta-analysis[J]. Medicina(Kaunas), 2022, 58( 7): 905. DOI: 10.3390/medicina58070905. |
| [6] |
BIGNA JJ, MODIYINJI AF, NANSSEU JR, et al. Burden of hepatitis E virus infection in pregnancy and maternofoetal outcomes: a systematic review and meta-analysis[J]. BMC Pregnancy Childbirth, 2020, 20( 1): 426. DOI: 10.1186/s12884-020-03116-2. |
| [7] |
AHMAD T, HUI J, MUSA TH, et al. Seroprevalence of hepatitis E virus infection in pregnant women: a systematic review and meta-analysis[J]. Ann Saudi Med, 2020, 40( 2): 136- 146. DOI: 10.5144/0256-4947.2020.136. |
| [8] |
SRIDHAR S, LO SK, XING F, et al. Clinical characteristics and molecular epidemiology of hepatitis E in Shenzhen, China: a shift toward foodborne transmission of hepatitis E virus infection[J]. Emerg Microbes Infect, 2017, 6( 12): e115. DOI: 10.1038/emi.2017.107. |
| [9] |
WANG L, LIU L, WEI Y, et al. Clinical and virological profiling of sporadic hepatitis E virus infection in China[J]. J Infect, 2016, 73( 3): 271- 279. DOI: 10.1016/j.jinf.2016.06.005. |
| [10] |
YUE N, WANG Q, ZHENG M, et al. Prevalence of hepatitis E virus infection among people and swine in mainland China: A systematic review and meta-analysis[J]. Zoonoses Public Health, 2019, 66( 3): 265- 275. DOI: 10.1111/zph.12555. |
| [11] |
BO QN, WANG SY, LI ZF, et al. Serological and clinical characteristics of hepatitis E virus infection in pregnant women in Qinhuangdao[J]. Chin Hepatol, 2019, 24( 10): 1150- 1154. DOI: 10.3969/j.issn.1008-1704.2019.10.019. |
| [12] |
BERGLØV A, HALLAGER S, WEIS N. Hepatitis E during pregnancy: Maternal and foetal case-fatality rates and adverse outcomes-A systematic review[J]. J Viral Hepat, 2019, 26( 11): 1240- 1248. DOI: 10.1111/jvh.13129. |
| [13] |
BHATNAGAR N, PRAKASH S, RAMAKRISHNA V, et al. Molecular characterisation of hepatitis E virus isolates from north India[J]. Indian J Med Microbiol, 2022, 40( 1): 91- 95. DOI: 10.1016/j.ijmmb.2021.09.004. |
| [14] |
SAYED IM, ABDELWAHAB SF. Is hepatitis E virus a neglected or emerging pathogen in Egypt?[J]. Pathogens, 2022, 11( 11): 1337. DOI: 10.3390/pathogens11111337. |
| [15] |
OKWARA VC, MBACHU II, NDUBUBA VI, et al. Seroprevalence, associated factors, and fetomaternal outcome in pregnant women that tested positive to hepatitis E antibodies in nigeria[J]. Obstet Gynecol Int, 2021, 2021: 9341974. DOI: 10.1155/2021/9341974. |
| [16] |
VELAVAN TP, PALLERLA SR, JOHNE R, et al. Hepatitis E: an update on one health and clinical medicine[J]. Liver Int, 2021, 41( 7): 1462- 1473. DOI: 10.1111/liv.14912. |
| [17] |
BOUTHRY E, BENACHI A, VIVANTI AJ, et al. Autochthonous hepatitis E during pregnancy, France[J]. Emerg Infect Dis, 2018, 24( 8): 1586- 1587. DOI: 10.3201/eid2408.180105. |
| [18] |
CHARRE C, RAMIÈRE C, DUMORTIER J, et al. Chronic genotype 3 hepatitis E in pregnant woman receiving infliximab and azathioprine[J]. Emerg Infect Dis, 2018, 24( 5): 941- 943. DOI: 10.3201/eid2405.171845. |
| [19] |
HUY PX, CHUNG DT, LINH DT, et al. Low prevalence of HEV infection and No associated risk of HEV transmission from mother to child among pregnant women in vietnam[J]. Pathogens, 2021, 10( 10): 1340. DOI: 10.3390/pathogens10101340. |
| [20] |
LI M, BU Q, GONG W, et al. Hepatitis E virus infection and its associated adverse feto-maternal outcomes among pregnant women in Qinhuangdao, China[J]. J Matern Fetal Neonatal Med, 2020, 33( 21): 3647- 3651. DOI: 10.1080/14767058.2019.1582630. |
| [21] |
ZHANG F, WANG J, CHENG J, et al. Clinical features of sporadic hepatitis E virus infection in pregnant women in Shanghai, China[J]. J Infect, 2022, 84( 1): 64- 70. DOI: 10.1016/j.jinf.2021.11.004. |
| [22] |
|
| [23] |
WU J, GUO Y, LU X, et al. Th1/Th2 cells and associated cytokines in acute hepatitis E and related acute liver failure[J]. J Immunol Res, 2020, 2020: 6027361. DOI: 10.1155/2020/6027361. |
| [24] |
LIN S, ZHANG YJ. Advances in hepatitis E virus biology and pathogenesis[J]. Viruses, 2021, 13( 2). DOI: 10.3390/v13020267. |
| [25] |
SHATA MTM, HETTA HF, SHARMA Y, et al. Viral hepatitis in pregnancy[J]. J Viral Hepat, 2022, 29( 10): 844- 861. DOI: 10.1111/jvh.13725. |
| [26] |
KHUROO MS. Hepatitis E and pregnancy: an unholy alliance unmasked from Kashmir, India[J]. Viruses, 2021, 13( 7): 1329. DOI: 10.3390/v13071329. |
| [27] |
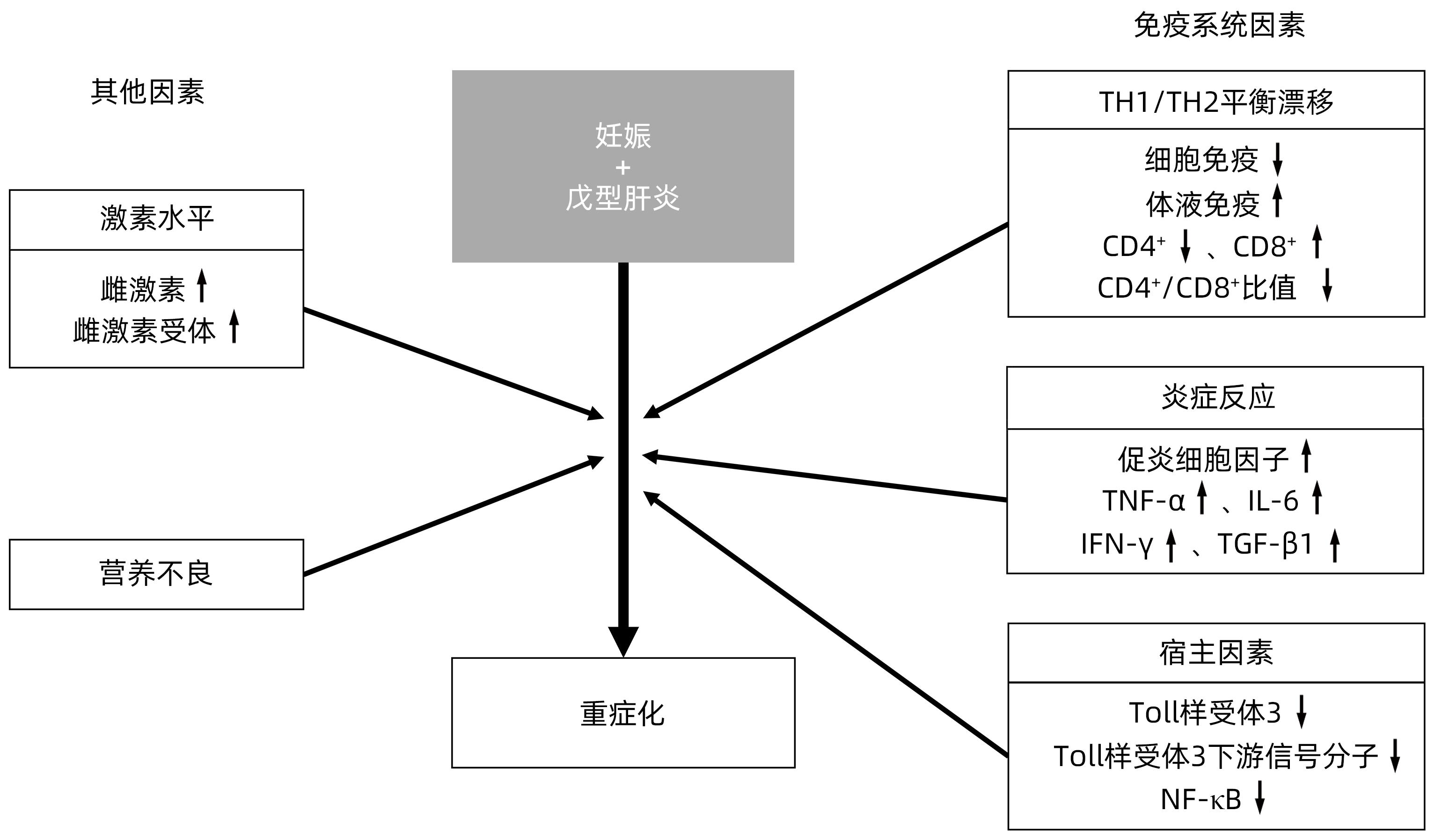
SAXENA A, SIDDIQUI H, SEVAK JK, et al. IL-7 induced CD4+ memory stem T cell-derived pro-inflammatory cytokine storm plays a role in hepatitis E induced pathogenesis in pregnant women[C]. AASLD, 2022: 1014.
|
| [28] |
HORVATITS T, PISCHKE S. HEV in pregnancy: Understanding the crucial role of steroid hormones[J]. Liver Int, 2019, 39( 4): 621- 622. DOI: 10.1111/liv.13942. |
| [29] |
SINGH S, DAGA MK, KUMAR A, et al. Role of oestrogen and its receptors in HEV-associated feto-maternal outcomes[J]. Liver Int, 2019, 39( 4): 633- 639. DOI: 10.1111/liv.13928. |
| [30] |
KUMAR A, SHARMA S, KAR P, et al. Impact of maternal nutrition in hepatitis E infection in pregnancy[J]. Arch Gynecol Obstet, 2017, 296( 5): 885- 895. DOI: 10.1007/s00404-017-4501-y. |
| [31] |
Chinese Society of Hepatology, Chinese Medical Association. Consensus on prevention and treatment of hepatitis E[J]. Chin J Hepatol, 2022, 30( 8): 820- 831. DOI: 10.3760/cma.j.cn501113-20220729-00401. |
| [32] |
WU J, BORTOLANZA M, ZHAI G, et al. Gut microbiota dysbiosis associated with plasma levels of Interferon-γ and viral load in patients with acute hepatitis E infection[J]. J Med Virol, 2022, 94( 2): 692- 702. DOI: 10.1002/jmv.27356. |
| [33] |
WU J, XU Y, CUI Y, et al. Dynamic changes of serum metabolites associated with infection and severity of patients with acute hepatitis E infection[J]. J Med Virol, 2022, 94( 6): 2714- 2726. DOI: 10.1002/jmv.27669. |
| [34] |
WU J, GUO N, ZHANG X, et al. HEV-LFS: A novel scoring model for patients with hepatitis E virus-related liver failure[J]. J Viral Hepat, 2019, 26( 11): 1334- 1343. DOI: 10.1111/jvh.13174. |
| [35] |
WU J, SHI C, SHENG X, et al. Prognostic nomogram for patients with hepatitis E virus-related acute liver failure: a multicenter study in China[J]. J Clin Transl Hepatol, 2021, 9( 6): 828- 837. DOI: 10.14218/JCTH.2020.00117. |
| [36] |
RIVERO-JUAREZ A, LOPEZ-LOPEZ P, PINEDA JA, et al. Limited value of single sampling for igm antibody determination as a diagnostic approach for acute hepatitis E virus infection[J]. Microbiol Spectr, 2021, 9( 1): e0038221. DOI: 10.1128/Spectrum.00382-21. |
| [37] |
AL-SADEQ DW, MAJDALAWIEH AF, MESLEH AG, et al. Laboratory challenges in the diagnosis of hepatitis E virus[J]. J Med Microbiol, 2018, 67( 4): 466- 480. DOI: 10.1099/jmm.0.000706. |
| [38] |
RIVEIRO-BARCIELA M, RANDO-SEGURA A, BARREIRA-DÍAZ A, et al. Unexpected long-lasting anti-HEV IgM positivity: Is HEV antigen a better serological marker for hepatitis E infection diagnosis?[J]. J Viral Hepat, 2020, 27( 7): 747- 753. DOI: 10.1111/jvh.13285. |
| [39] |
MARION O, CAPELLI N, LHOMME S, et al. Hepatitis E virus genotype 3 and capsid protein in the blood and urine of immunocompromised patients[J]. J Infect, 2019, 78( 3): 232- 240. DOI: 10.1016/j.jinf.2019.01.004. |
| [40] |
YING D, HE Q, TIAN W, et al. Urine is a viral antigen reservoir in hepatitis E virus infection[J]. Hepatology, 2023, 77( 5): 1722- 1734. DOI: 10.1002/hep.32745. |







 DownLoad:
DownLoad: