| [1] |
|
| [2] |
|
| [3] |
HOOFNAGLE JH, NELSON KE, PURCELL RH. Hepatitis E[J]. N Engl J Med, 2012, 367( 13): 1237- 1244. DOI: 10.1056/nejmra1204512. |
| [4] |
MA ZR, DE MAN RA, KAMAR N, et al. Chronic hepatitis E: Advancing research and patient care[J]. J Hepatol, 2022, 77( 4): 1109- 1123. DOI: 10.1016/j.jhep.2022.05.006. |
| [5] |
SMITH DB, IZOPET J, NICOT F, et al. Update: Proposed reference sequences for subtypes of hepatitis E virus(species Orthohepevirus A)[J]. J Gen Virol, 2020, 101( 7): 692- 698. DOI: 10.1099/jgv.0.001435. |
| [6] |
ASLAN AT, BALABAN HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment[J]. World J Gastroenterol, 2020, 26( 37): 5543- 5560. DOI: 10.3748/wjg.v26.i37.5543. |
| [7] |
PISCHKE S, WEDEMEYER H. Hepatitis E virus infection: Multiple faces of an underestimated problem[J]. J Hepatol, 2013, 58( 5): 1045- 1046. DOI: 10.1016/j.jhep.2012.12.013. |
| [8] |
KAMAR N, SELVES J, MANSUY JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients[J]. N Engl J Med, 2008, 358( 8): 811- 817. DOI: 10.1056/nejmoa0706992. |
| [9] |
DALTON HR, BENDALL RP, KEANE FE, et al. Persistent carriage of hepatitis E virus in patients with HIV infection[J]. N Engl J Med, 2009, 361( 10): 1025- 1027. DOI: 10.1056/NEJMc0903778. |
| [10] |
VON FELDEN J, ALRIC L, PISCHKE S, et al. The burden of hepatitis E among patients with haematological malignancies: A retrospective European cohort study[J]. J Hepatol, 2019, 71( 3): 465- 472. DOI: 10.1016/j.jhep.2019.04.022. |
| [11] |
KAMAR N, GARROUSTE C, HAAGSMA EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants[J]. Gastroenterology, 2011, 140( 5): 1481- 1489. DOI: 10.1053/j.gastro.2011.02.050. |
| [12] |
GETSUWAN S, PASOMSUB E, YUTTHANAKARNWIKOM P, et al. Seroprevalence of hepatitis E virus after pediatric liver transplantation[J]. J Trop Pediatr, 2023, 69( 2): fmad011. DOI: 10.1093/tropej/fmad011. |
| [13] |
LHOMME S, LEGRAND-ABRAVANEL F, KAMAR N, et al. Screening, diagnosis and risks associated with Hepatitis E virus infection[J]. Expert Rev Anti Infect Ther, 2019, 17( 6): 403- 418. DOI: 10.1080/14787210.2019.1613889. |
| [14] |
LAMPEJO T, CURTIS C, IJAZ S, et al. Nosocomial transmission of hepatitis E virus and development of chronic infection: The wider impact of COVID-19[J]. J Clin Virol, 2022, 148: 105083. DOI: 10.1016/j.jcv.2022.105083. |
| [15] |
GENG YS, ZHANG HX, HUANG WJ, et al. Persistent hepatitis e virus genotype 4 infection in a child with acute lymphoblastic leukemia[J]. Hepat Mon, 2014, 14( 1): e15618. DOI: 10.5812/hepatmon.15618. |
| [16] |
LENGLART A, CHAPPÉ C, GRULOIS I, et al. Hepatitis E virus infection in pediatric oncology[J]. J Pediatr Hematol Oncol, 2023, 45( 1): e150- e153. DOI: 10.1097/MPH.0000000000002539. |
| [17] |
European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection[J]. J Hepatol, 2012, 57( 1): 167- 185.
|
| [18] |
NASIR M, WU GY. HEV and HBV dual infection: A review[J]. J Clin Transl Hepatol, 2020, 8( 3): 313- 321. DOI: 10.14218/JCTH.2020.00030. |
| [19] |
XIONG LS, CUI SF, ZHOU JG, et al. Detection and analysis of HAV-HEV, HGV infection in patients with viral hepatitis[J]. Chin J Hepatol, 2004, 12( 7): 395- 396. DOI: 10.3760/j.issn: 1007-3418.2004.07.004.
|
| [20] |
TSENG TC, LIU CJ, CHANG CT, et al. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis[J]. J Hepatol, 2020, 72( 6): 1105- 1111. DOI: 10.1016/j.jhep.2020.01.012. |
| [21] |
ZHAO H, YE WY, YU X, et al. Hepatitis E virus superinfection impairs long-term outcome in hospitalized patients with hepatitis B virus-related decompensated liver cirrhosis[J]. Ann Hepatol, 2023, 28( 2): 100878. DOI: 10.1016/j.aohep.2022.100878. |
| [22] |
CHOW CW, TSANG SWC, TSANG OTY, et al. Comparison of acute hepatitis E infection outcome in patients with and without chronic hepatitis B infection: A 10 year retrospective study in three regional hospitals in Hong Kong[J]. J Clin Virol, 2014, 60( 1): 4- 10. DOI: 10.1016/j.jcv.2014.01.024. |
| [23] |
CHEN C, ZHANG SY, ZHANG DD, et al. Clinical features of acute hepatitis E super-infections on chronic hepatitis B[J]. World J Gastroenterol, 2016, 22( 47): 10388- 10397. DOI: 10.3748/wjg.v22.i47.10388. |
| [24] |
JILANI N, DAS BC, HUSAIN SA, et al. Hepatitis E virus infection and fulminant hepatic failure during pregnancy[J]. J Gastroenterol Hepatol, 2007, 22( 5): 676- 682. DOI: 10.1111/j.1440-1746.2007.04913.x. |
| [25] |
KHUROO MS, Aetiology KAMILI S., clinical course and outcome of sporadic acute viral hepatitis in pregnancy[J]. J Viral Hepat, 2003, 10( 1): 61- 69. DOI: 10.1046/j.1365-2893.2003.00398.x. |
| [26] |
DI BARTOLOMEO S, CARUBBI F, CIPRIANI P. Hepatitis E virus and rheumatic diseases: What do rheumatologists need to know?[J]. BMC Rheumatol, 2020, 4: 51. DOI: 10.1186/s41927-020-00149-0. |
| [27] |
KAR P, SENGUPTA A. A guide to the management of hepatitis E infection during pregnancy[J]. Expert Rev Gastroenterol Hepatol, 2019, 13( 3): 205- 211. DOI: 10.1080/17474124.2019.1568869. |
| [28] |
BOSE PD, DAS BC, KUMAR A, et al. High viral load and deregulation of the progesterone receptor signaling pathway: Association with hepatitis E-related poor pregnancy outcome[J]. J Hepatol, 2011, 54( 6): 1107- 1113. DOI: 10.1016/j.jhep.2010.08.037. |
| [29] |
GOUILLY J, CHEN Q, SIEWIERA J, et al. Genotype specific pathogenicity of hepatitis E virus at the human maternal-fetal interface[J]. Nat Commun, 2018, 9( 1): 4748. DOI: 10.1038/s41467-018-07200-2. |
| [30] |
BHATIA V, SINGHAL A, PANDA SK, et al. A 20-year single-center experience with acute liver failure during pregnancy: Is the prognosis really worse?[J]. Hepatology, 2008, 48( 5): 1577- 1585. DOI: 10.1002/hep.22493. |
| [31] |
SHARMA S, KUMAR A, KAR P, et al. Risk factors for vertical transmission of hepatitis E virus infection[J]. J Viral Hepat, 2017, 24( 11): 1067- 1075. DOI: 10.1111/jvh.12730. |
| [32] |
RIVERO-JUAREZ A, FRIAS M, RODRIGUEZ-CANO D, et al. Isolation of hepatitis E virus from breast milk during acute infection[J]. Clin Infect Dis, 2016, 62( 11): 1464. DOI: 10.1093/cid/ciw186. |
| [33] |
Chinese Society of Hepatology, Chinese Medical Association. Consensus on prevention and treatment of hepatitis E[J]. Chin J Hepatol, 2022, 30( 8): 820- 831. DOI: 10.3760/cma.j.cn501113-20220729-00401. |
| [34] |
CORDES AK, GOUDEVA L, LÜTGEHETMANN M, et al. Risk of transfusion-transmitted hepatitis E virus infection from pool-tested platelets and plasma[J]. J Hepatol, 2022, 76( 1): 46- 52. DOI: 10.1016/j.jhep.2021.08.018. |
| [35] |
BOLAND F, MARTINEZ A, POMEROY L, et al. Blood donor screening for hepatitis E virus in the European union[J]. Transfus Med Hemother, 2019, 46( 2): 95- 103. DOI: 10.1159/000499121. |
| [36] |
ZHU FC, ZHANG J, ZHANG XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial[J]. Lancet, 2010, 376( 9744): 895- 902. DOI: 10.1016/S0140-6736(10)61030-6. |
| [37] |
ZHANG J, ZHANG XF, HUANG SJ, et al. Long-term efficacy of a hepatitis E vaccine[J]. N Engl J Med, 2015, 372( 10): 914- 922. DOI: 10.1056/NEJMoa1406011. |
| [38] |
ØVERBØ J, AZIZ A, ZAMAN K, et al. Immunogenicity and safety of a two-dose regimen with hepatitis E virus vaccine in healthy adults in rural Bangladesh: A randomized, double-blind, controlled, phase 2/pilot trial[J]. Vaccine, 2023, 41( 5): 1059- 1066. DOI: 10.1016/j.vaccine.2022.12.064. |
| [39] |
WANG KH, ZHOU LZ, ZHANG X, et al. Hepatitis E vaccine candidate harboring a non-particulate immunogen of E2 fused with CRM197 fragment A[J]. Antiviral Res, 2019, 164: 154- 161. DOI: 10.1016/j.antiviral.2019.02.013. |
| [40] |
GU Y, TANG XH, ZHANG X, et al. Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody[J]. Cell Res, 2015, 25( 5): 604- 620. DOI: 10.1038/cr.2015.34. |
| [41] |
CHEN ZH, WEI JF, JIANG L, et al. Case Report: Chronic hepatitis E in a hematopoietic stem cell transplant recipient: The first report of hepatitis E virus genotype 4 causing chronic infection in a non-solid organ recipient[J]. Front Immunol, 2022, 13: 954697. DOI: 10.3389/fimmu.2022.954697. |
| [42] |
ZHANG HY, RAO HY, WANG YJ, et al. Evaluation of an antigen assay for diagnosing acute and chronic hepatitis E genotype 4 infection[J]. J Gastroenterol Hepatol, 2019, 34( 2): 458- 465. DOI: 10.1111/jgh.14405. |
| [43] |
ANKCORN M, SAID B, MORGAN D, et al. Persistent hepatitis E virus infection across England and Wales 2009-2017: Demography, virology and outcomes[J]. J Viral Hepat, 2021, 28( 2): 420- 430. DOI: 10.1111/jvh.13424. |
| [44] |
KAMAR N, ROSTAING L, ABRAVANEL F, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation[J]. Clin Infect Dis, 2010, 50( 5): e30- e33. DOI: 10.1086/650488. |
| [45] |
GALLACHER J, TAHA Y, SILVA FILIPE A DA, et al. Ledipasvir/sofosbuvir and ribavirin for the treatment of ribavirin-refractory persistent hepatitis E virus infection[J]. IDCases, 2023, 32: e01741. DOI: 10.1016/j.idcr.2023.e01741. |
| [46] |
CORNBERG M, PISCHKE S, MÜLLER T, et al. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E-The HepNet SofE pilot study[J]. J Hepatol, 2020, 73( 3): 696- 699. DOI: 10.1016/j.jhep.2020.05.020. |
| [47] |
TAVITIAN S, PERON JM, HUGUET F, et al. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies[J]. Emerg Infect Dis, 2015, 21( 8): 1466- 1469. DOI: 10.3201/eid2108.150199. |
| [48] |
ZHANG F, XU LD, ZHANG Q, et al. Targeting proteostasis of the HEV replicase to combat infection in preclinical models[J]. J Hepatol, 2023, 78( 4): 704- 716. DOI: 10.1016/j.jhep.2022.12.010. |
| [49] |
KINAST V, BURKARD TL, TODT D, et al. Hepatitis E virus drug development[J]. Viruses, 2019, 11( 6): 485. DOI: 10.3390/v11060485. |



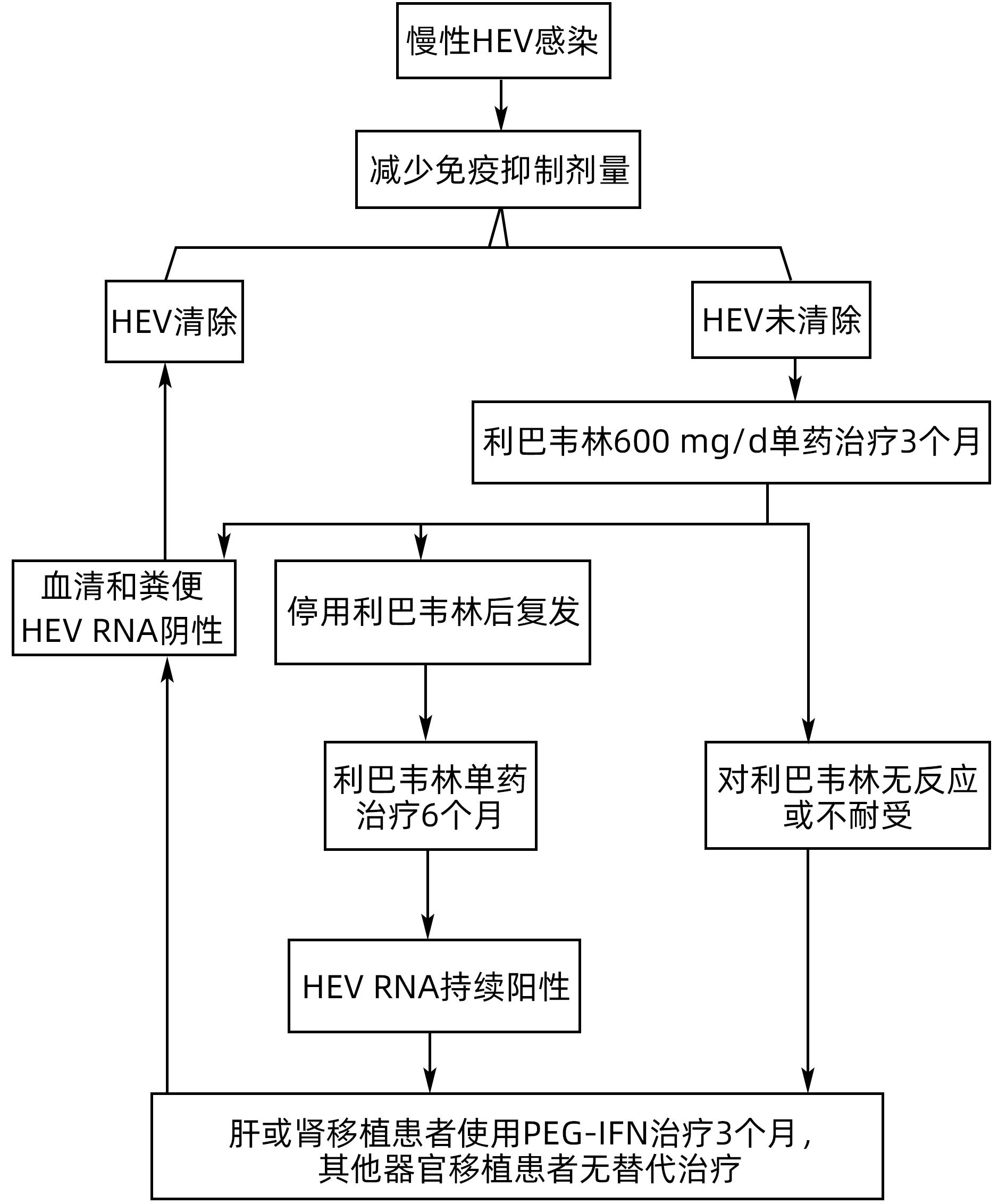




 DownLoad:
DownLoad: