| [1] |
LI Q, TAN Y, CHEN SN, et al. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling[J]. J Recept Signal Transduct Res, 2021, 41( 3): 294- 303. DOI: 10.1080/10799893.2020.1808675. |
| [2] |
|
| [3] |
CARPINO G, DEL BEN M, PASTORI D, et al. Increased liver localization of lipopolysaccharides in human and experimental NAFLD[J]. Hepatology, 2020, 72( 2): 470- 485. DOI: 10.1002/hep.31056. |
| [4] |
JING ZT, LIU W, XUE CR, et al. AKT activator SC79 protects hepatocytes from TNF-α-mediated apoptosis and alleviates d-Gal/LPS-induced liver injury[J]. Am J Physiol Gastrointest Liver Physiol, 2019, 316( 3): G387- G396. DOI: 10.1152/ajpgi.00350.2018. |
| [5] |
YAN CY, OUYANG SH, WANG X, et al. Celastrol ameliorates Propionibacterium acnes/LPS-induced liver damage and MSU-induced gouty arthritis via inhibiting K63 deubiquitination of NLRP3[J]. Phytomedicine, 2021, 80: 153398. DOI: 10.1016/j.phymed.2020.153398. |
| [6] |
CIESIELSKA A, MATYJEK M, KWIATKOWSKA K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling[J]. Cell Mol Life Sci, 2021, 78( 4): 1233- 1261. DOI: 10.1007/s00018-020-03656-y. |
| [7] |
ISHIDA K, KAJI K, SATO S, et al. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway[J]. J Nutr Biochem, 2021, 89: 108573. DOI: 10.1016/j.jnutbio.2020.108573. |
| [8] |
|
| [9] |
YANG WC, WANG YX, ZHANG CG, et al. Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation[J]. Front Pharmacol, 2022, 13: 865689. DOI: 10.3389/fphar.2022.865689. |
| [10] |
ZHANG HB, YUAN B, HUANG HF, et al. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H 2O 2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation[J]. Braz J Med Biol Res, 2018, 51( 10): e7439. DOI: 10.1590/1414-431x20187439. |
| [11] |
FOROUZANFAR F, ASADPOUR E, HOSSEINZADEH H, et al. Safranal protects against ischemia-induced PC12 cell injury through inhibiting oxidative stress and apoptosis[J]. Naunyn Schmiedebergs Arch Pharmacol, 2021, 394( 4): 707- 716. DOI: 10.1007/s00210-020-01999-8. |
| [12] |
HOSSEINI A, RAZAVI BM, HOSSEINZADEH H. Pharmacokinetic properties of saffron and its active components[J]. Eur J Drug Metab Pharmacokinet, 2018, 43( 4): 383- 390. DOI: 10.1007/s13318-017-0449-3. |
| [13] |
NANDA SJ, MADAN K. The role of Safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: A systematic review[J]. Heliyon, 2021, 7( 2): e06117. DOI: 10.1016/j.heliyon.2021.e06117. |
| [14] |
LOO DT. TUNEL assay: An overview of techniques[M]// In Situ Detection of DNA Damage. New Jersey: Humana Press, 2003: 21- 30. DOI: 10.1385/1-59259-179-5: 21.
|
| [15] |
LI QH, ZHAO QW. Effect of continuous blood purification on inflammatory factors and vascular endothelial permeability in sepsis patients with acute respiratory distress syndrome[J]. Trauma Crit Care Med, 2021, 9( 4): 304- 306. DOI: 10.16048/j.issn.2095-5561.2021.04.16. |
| [16] |
ZUO HZ, TIAN LJ, YANG XF, et al. Study on Logistic regression analysis of risk factors for secondary acute kidney injury in patients with sepsis[J]. J Changchun Univ Chin Med, 2023, 39( 8): 915- 920. DOI: 10.13463/j.cnki.cczyy.2023.08.021. |
| [17] |
LELUBRE C, VINCENT JL. Mechanisms and treatment of organ failure in sepsis[J]. Nat Rev Nephrol, 2018, 14( 7): 417- 427. DOI: 10.1038/s41581-018-0005-7. |
| [18] |
WU Y, REN JA. Role of oxidative stress in liver injury caused by sepsis[J]. Chin J Pact Surg, 2014, 34( 2): 187- 189.
吴吟, 任建安. 氧化应激在脓毒症导致的肝损伤中的作用[J]. 中国实用外科杂志, 2014, 34( 2): 187- 189.
|
| [19] |
CERDÁ-BERNAD D, VALERO-CASES E, PASTOR JJ, et al. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action[J]. Crit Rev Food Sci Nutr, 2022, 62( 12): 3232- 3249. DOI: 10.1080/10408398.2020.1864279. |
| [20] |
ABDALLA Y, ABDALLA A, HAMZA AA, et al. Safranal prevents liver cancer through inhibiting oxidative stress and alleviating inflammation[J]. Front Pharmacol, 2022, 12: 777500. DOI: 10.3389/fphar.2021.777500. |
| [21] |
GUPTA M, WANI A, AHSAN AU, et al. Safranal inhibits NLRP3 inflammasome activation by preventing ASC oligomerization[J]. Toxicol Appl Pharmacol, 2021, 423: 115582. DOI: 10.1016/j.taap.2021.115582. |
| [22] |
ABDALLA A, MURALI C, AMIN A. Safranal inhibits angiogenesis via targeting HIF-1α/VEGF machinery: in vitro and Ex vivo insights[J]. Front Oncol, 2022, 11: 789172. DOI: 10.3389/fonc.2021.789172. |
| [23] |
XIAO Q, SUN YY, LU ZJ, et al. Protective effects of safranal on diabetic retinopathy in human microvascular endothelial cells and related pathways analyzed with transcriptome sequencing[J]. Front Endocrinol, 2022, 13: 945446. DOI: 10.3389/fendo.2022.945446. |
| [24] |
ÖN ALAYUNT, AKSOY L, KARAFAKIOĞLU YS, et al. Assessment of anti-inflammatory and antioxidant properties of safranal on CCl 4-induced oxidative stress and inflammation in rats[J]. An Acad Bras Cienc, 2019, 91( 2): e20181235. DOI: 10.1590/0001-3765201920181235. |
| [25] |
SABIR U, IRFAN HM, ALAMGEER, et al. Reduction of hepatic steatosis, oxidative stress, inflammation, ballooning and insulin resistance after therapy with safranal in NAFLD animal model: A new approach[J]. J Inflamm Res, 2022, 15: 1293- 1316. DOI: 10.2147/JIR.S354878. |
| [26] |
CAO LJ, GONG H, YAN M, et al. Research progress on Nrf2-ARE signaling pathway involved in liver disease pathological mechanism[J]. Chin Pharmacol Bull, 2015, 31( 8): 1057- 1061. DOI: 10.3969/j.issn.1001-1978.2015.08.006. |
| [27] |
LU XL, JIANG YY, CAO Q. The role of oxidative stress and nuclear factor erythroid 2-related factor 2 in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2020, 36( 4): 924- 927. DOI: 10.3969/j.issn.1001-5256.2020.04.048. |
| [28] |
WANG TT, CHEN CY, YANG L, et al. Role of Nrf2/HO-1 signal axis in the mechanisms for oxidative stress-relevant diseases[J]. J Cent South Univ Med Sci, 2019, 44( 1): 74- 80. DOI: 10.11817/j.issn.1672-7347.2019.01.012. |
| [29] |
LERTNIMITPHUN P, ZHANG WH, FU WW, et al. Safranal alleviated OVA-induced asthma model and inhibits mast cell activation[J]. Front Immunol, 2021, 12: 585595. DOI: 10.3389/fimmu.2021.585595. |
| [30] |
WANG HF, ZHENG B, CHE KM, et al. Protective effects of safranal on hypoxia/reoxygenation-induced injury in H9c2 cardiac myoblasts via the PI3K/AKT/GSK3β signaling pathway[J]. Exp Ther Med, 2021, 22( 6): 1400. DOI: 10.3892/etm.2021.10836. |
| [31] |
XUE YR, JIN WY, XUE YC, et al. Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca 2+ homeostasis[J]. J Pharmacol Sci, 2020, 143( 3): 156- 164. DOI: 10.1016/j.jphs.2020.03.005. |
| [32] |
ZHANG YB, ZHAO Y, GUO JY, et al. Anticancer activity of safranal against colon carcinoma is due to induction of apoptosis and G2/M cell cycle arrest mediated by suppression of mTOR/PI3K/Akt pathway[J]. J BUON, 2021, 26( 1): 297.
|



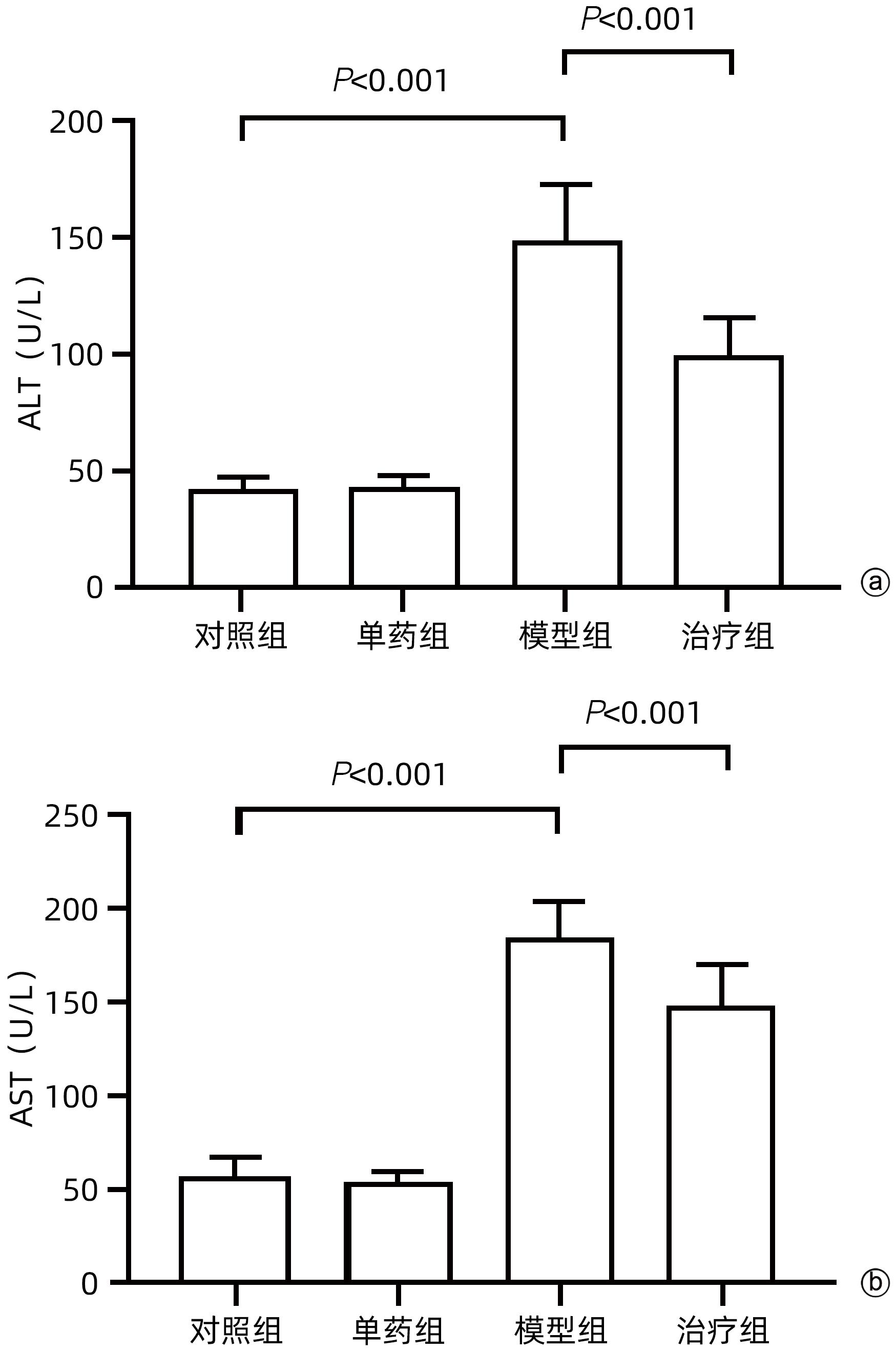




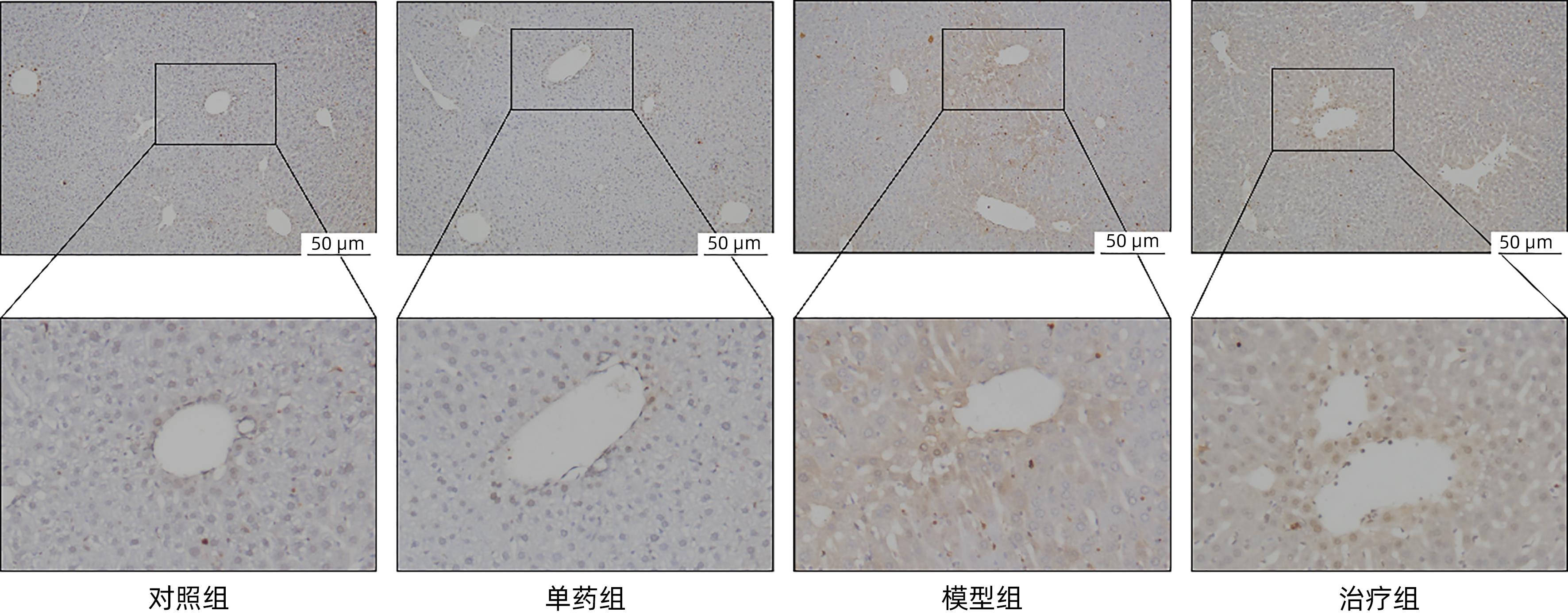
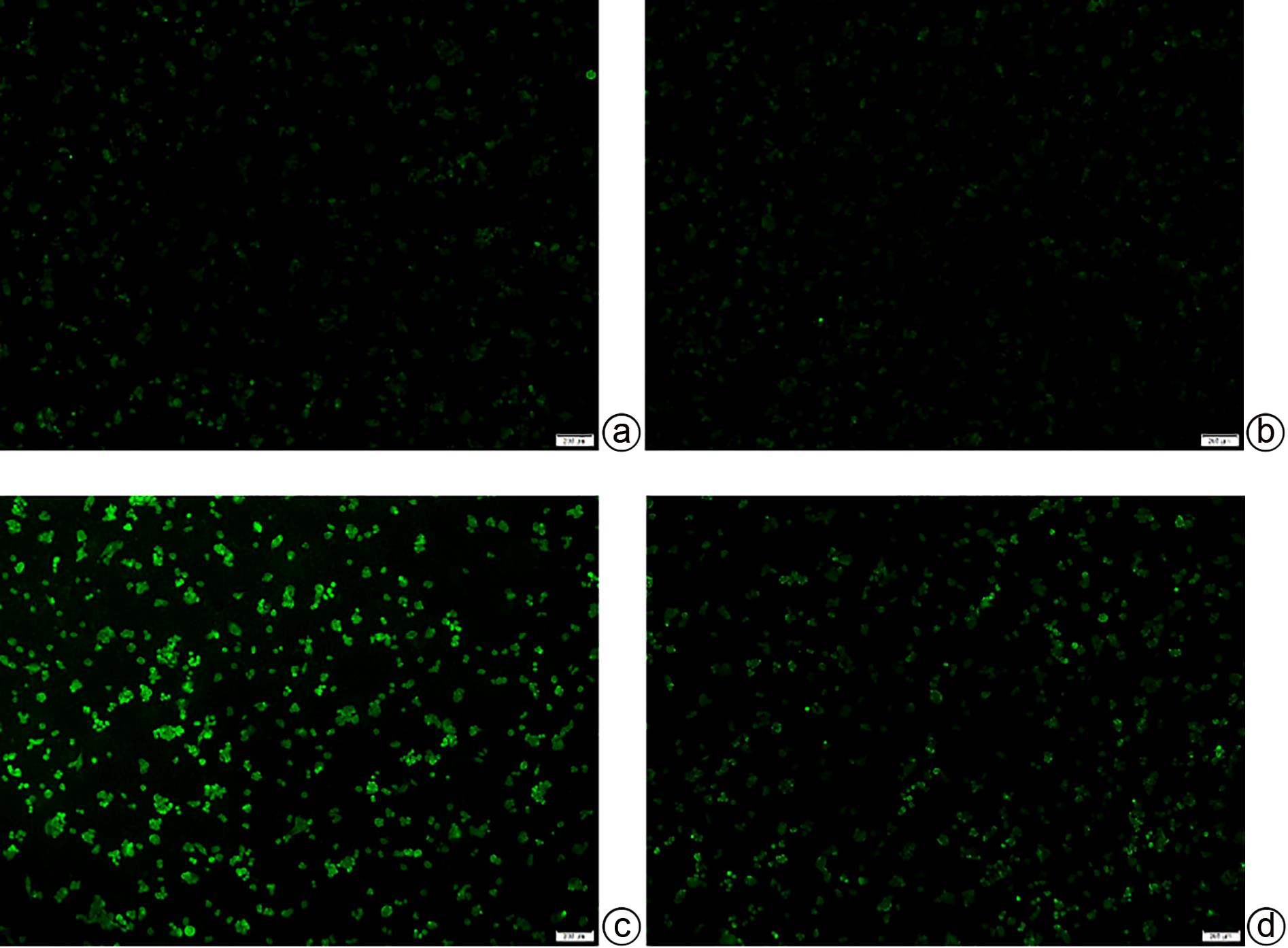
 DownLoad:
DownLoad: