| [1] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. |
| [2] |
PARK JW, CHEN M, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study[J]. Liver Int, 2015, 35( 9): 2155- 2166. DOI: 10.1111/liv.12818. |
| [3] |
KORDE LA, SOMERFIELD MR, CAREY LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline[J]. J Clin Oncol, 2021, 39( 13): 1485- 1505. DOI: 10.1200/JCO.20.03399. |
| [4] |
HANK T, BÜCHLER MW, NEOPTOLEMOS JP. Neoadjuvant chemotherapy in pancreatic cancer[J]. JAMA Surg, 2021, 156( 4): 397- 397. DOI: 10.1001/jamasurg.2020.6273. |
| [5] |
ROSEN G, MARCOVE R C, CAPARROS B, et al. Primary osteogenic sarcoma. The rationale for preoperative chemotherapy and delayed surgery[J]. Cancer, 1979, 43( 6): 2163- 2177. DOI: 3.0.co;2-s">10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s.
|
| [6] |
FREI E 3rd. Clinical cancer research: an embattled species[J]. Cancer, 1982, 50( 10): 1979- 1992. DOI: 3.0.co;2-d">10.1002/1097-0142(19821115)50:10<1979::aid-cncr2820501002>3.0.co;2-d.
|
| [7] |
TRIMBLE EL, UNGERLEIDER RS, ABRAMS JA, et al. Neoadjuvant therapy in cancer treatment[J]. Cancer, 1993, 72( 11 Suppl): 3515- 3524. DOI: 10.1002/1097-0142(19931201)72:11 +<3515::aid-cncr2820721619>3. 0.co;2-a. |
| [8] |
HWANG TL, CHEN MF, LEE TY, et al. Resection of hepatocellular carcinoma after transcatheter arterial embolization. Reevaluation of the advantages and disadvantages of preoperative embolization[J]. Arch Surg, 1987, 122( 7): 756- 759. DOI: 10.1001/archsurg.1987.01400190022004. |
| [9] |
General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. |
| [10] |
ZHOU H, SONG T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma[J]. Biosci Trends, 2021, 15( 3): 155- 160. DOI: 10.5582/bst.2021.01091. |
| [11] |
POON RT, FAN ST, NG IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma[J]. Cancer, 2000, 89( 3): 500- 507.
|
| [12] |
TSILIMIGRAS DI, BAGANTE F, MORIS D, et al. Recurrence patterns and outcomes after resection of hepatocellular carcinoma within and beyond the barcelona clinic liver cancer criteria[J]. Ann Surg Oncol, 2020, 27( 7): 2321- 2331. DOI: 10.1245/s10434-020-08452-3. |
| [13] |
ZHAO H, HUA Y, DAI T, et al. Development and validation of a novel predictive scoring model for microvascular invasion in patients with hepatocellular carcinoma[J]. Eur J Radiol, 2017, 88: 32- 40. DOI: 10.1016/j.ejrad.2016.12.030. |
| [14] |
von FELDEN J, HEIM D, SCHULZE K, et al. High expression of micro RNA-135A in hepatocellular carcinoma is associated with recurrence within 12 months after resection[J]. BMC Cancer, 2017, 17( 1): 60. DOI: 10.1186/s12885-017-3053-7. |
| [15] |
LIU T, TAN J, WU M, et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma(HCC) activity by activating CD39 + CD8 + T cells[J]. Gut, 2021, 70( 10): 1965- 1977. DOI: 10.1136/gutjnl-2020-322196. |
| [16] |
HARDING JJ, NANDAKUMAR S, ARMENIA J, et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies[J]. Clin Cancer Res, 2019, 25( 7): 2116- 2126. DOI: 10.1158/1078-0432.CCR-18-2293. |
| [17] |
HORWITZ E, STEIN I, ANDREOZZI M, et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment[J]. Cancer Discov, 2014, 4( 6): 730- 743. DOI: 10.1158/2159-8290.CD-13-0782. |
| [18] |
ZHANG Z, LIU Q, HE J, et al. The effect of preoperative transcatheter hepatic arterial chemoembolization on disease-free survival after hepatectomy for hepatocellular carcinoma[J]. Cancer, 2000, 89( 12): 2606- 2612.
|
| [19] |
GERUNDA GE, NERI D, MERENDA R, et al. Role of transarterial chemoembolization before liver resection for hepatocarcinoma[J]. Liver Transpl, 2000, 6( 5): 619- 626. DOI: 10.1053/jlts.2000.8312. |
| [20] |
CHUA TC, LIAUW W, SAXENA A, et al. Systematic review of neoadjuvant transarterial chemoembolization for resectable hepatocellular carcinoma[J]. Liver Int, 2010, 30( 2): 166- 174. DOI: 10.1111/j.1478-3231.2009.02166.x. |
| [21] |
QADAN M, FONG ZV, DELMAN AM, et al. Review of use of Y90 as a bridge to liver resection and transplantation in hepatocellular carcinoma[J]. J Gastrointest Surg, 2021, 25: 2690- 2699. DOI: 10.1007/s11605-021-05095-x. |
| [22] |
SALEM R, JOHNSON GE, KIM E, et al. Yttrium‐90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study[J]. Hepatology, 2021, 74( 5): 2342- 2352. DOI: 10.1002/hep.31819. |
| [23] |
KOKABI N, CAMACHO JC, XING M, et al. Open‐label prospective study of the safety and efficacy of glass‐based yttrium 90 radioembolization for infiltrative hepatocellular carcinoma with portal vein thrombosis[J]. Cancer, 2015, 121( 13): 2164- 2174. DOI: 10.1002/cncr.29275. |
| [24] |
WEI X, JIANG Y, ZHANG X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study[J]. J Clin Oncol, 2019, 37( 24): 2141. DOI: 10.1200/JCO.18.02184. |
| [25] |
KAMIYAMA T, NAKANISHI K, YOKOO H, et al. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma[J]. Int J Clin Oncol, 2007, 12( 5): 363- 368. DOI: 10.1007/s10147-007-0701-y. |
| [26] |
LI Q J, HE M K, CHEN H W, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase Ⅲ trial[J]. J Clin Oncol, 2022, 40( 2): 150- 160. DOI: 10.1200/JCO.21.00608. |
| [27] |
HE MK, LE Y, LI QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study[J]. Chin J Cancer, 2017, 36( 1): 83. DOI: 10.1186/s40880-017-0251-2. |
| [28] |
LYU N, WANG X, LI J B, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase Ⅲ trial(FOHAIC-1)[J]. J Clin Oncol, 2022, 40( 5): 468- 480. DOI: 10.1200/JCO.21.01963. |
| [29] |
HUANG J, HUANG W, ZHAN M, et al. Drug-eluting bead transarterial chemoembolization combined with FOLFOX-based hepatic arterial infusion chemotherapy for large or huge hepatocellular carcinoma[J]. J Hepatocell Carcinoma, 2021, 8: 1445- 1458. DOI: 10.2147/JHC.S339379. |
| [30] |
BYUN HK, KIM HJ, IM YR, et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma[J]. Radiother Oncol, 2019, 133: 1- 8. DOI: 10.1016/j.radonc.2018.12.025. |
| [31] |
BARBIER L, FUKS D, PESSAUX P, et al. Safety of liver resection for hepatocellular carcinoma after sorafenib therapy: a multicenter case-matched study[J]. Ann Surg Oncol, 2013, 20( 11): 3603- 3609. DOI: 10.1245/s10434-013-3029-z. |
| [32] |
KUDO M, FINN R S, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391( 10126): 1163- 1173. DOI: 10.1016/S0140-6736(18)30207-1. |
| [33] |
TADA M, ICHIDA A, ARITA J, et al. Multicenter prospective study to evaluate the efficacy of lenvatinib to achieve conversion surgery for initially unresectable hepatocellular carcinoma: LENS-HCC trial[J]. J Clin Oncol, 2022, 40( 4): 458. DOI: 10.1200/JCO.2022.40.4_suppl.458. |
| [34] |
YANG XR, SUN HC, XIE Q, et al. Chinese expert guidance on overall application of lenvatinib in hepatocellular carcinoma[J]. Chin J Dig Surg, 2023, 22( 2): 167- 180. DOI: 10.3760/cma.j.cn115610-20230201-00035. |
| [35] |
LIN YY, TAN CT, CHEN CW, et al. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: implication for the future of immunotherapy[J]. Semin Liver Dis, 2018, 38( 4): 379- 388. DOI: 10.1055/s-0038-1673621. |
| [36] |
LIANG J, LI L. Progress and consideration of immunotherapy strategy for hepatocellular carcinoma[J]. Chin J Dig Surg, 2021, 20( 2): 184- 190. DOI: 10.3760/cma.j.cn115610-20201228-00809. |
| [37] |
HO WJ, ZHU Q, DURHAM J, et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity[J]. Nat Cancer, 2021, 2( 9): 891- 903. DOI: 10.1038/s43018-021-00234-4. |
| [38] |
LIU J, BLAKE SJ, YONG MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease[J]. Cancer Discov, 2016, 6( 12): 1382- 1399. DOI: 10.1158/2159-8290.CD-16-0577. |
| [39] |
MARRON TU, FIEL MI, HAMON P, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial[J]. Lancet Gastroenterol Hepato, 2022, 7( 3): 219- 229. DOI: 10.1016/S2468-1253(21)00385-X. |
| [40] |
KASEB AO, HASANOV E, CAO H, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial[J]. Lancet Gastroenterol Hepatol, 2022, 7( 3): 208- 218. DOI: 10.1016/S2468-1253(21)00427-1. |
| [41] |
RAHMA OE, HODI FS. The intersection between tumor angiogenesis and immune suppression[J]. Clin Cancer Res, 2019, 25( 18): 5449- 5457. DOI: 10.1158/1078-0432.CCR-18-1543. |
| [42] |
ZHANG N, LIU XL, XU ZQ, et al. Research progress in relationship between tumor anti-angiogenesis and immunotherapy[J]. J Jilin Univ(Medicine Edition), 2021, 47( 4): 1056- 1063. DOI: 10.13481/j.1671-587X.20210434. |
| [43] |
XIA Y, WANG P, PU L, et al. Preliminary efficacy and safety of perioperative treatment of camrelizumab combined with apatinib in resectable hepatocellular carcinoma(HCC): A prospective phase Ⅱ study[J]. J Clin Oncol, 2021, 39( 15_Suppl): 4082. DOI: 10.1200/JCO.2021.39.15_suppl.4082. |
| [44] |
XU L, ZHANG Y, WANG X, et al. Transarterial infusion chemotherapy(TAI) combined with Sintilimab in locally advanced, potentially resectable hepatocellular carcinoma(HCC)[J]. J Clin Oncol, 2020, 38( 15_Suppl): e16593. DOI: 10.1200/JCO.2020.38.15_suppl.e16593. |
| [45] |
LLOVET JM, VILLANUEVA A, MARRERO JA, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference[J]. Hepatology, 2021, 73: 158- 191. DOI: 10.1002/hep.31327. |
| [46] |
KHAN KA, KERBEL RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa[J]. Nat Rev Clin Oncol, 2018, 15( 5): 310- 324. DOI: 10.1038/nrclinonc.2018.9. |
| [47] |
COTTRELL TR, THOMPSON ED, FORDE PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria(irPRC)[J]. Ann Oncol, 2018, 29( 8): 1853- 1860. DOI: 10.1093/annonc/mdy218. |
| [48] |
SASAKI A, IWASHITA Y, SHIBATA K, et al. Preoperative transcatheter arterial chemoembolization reduces long-term survival rate after hepatic resection for resectable hepatocellular carcinoma[J]. Eur J Surg Oncol, 2006, 32( 7): 773- 779. DOI: 10.1016/j.ejso.2006.04.002. |
| [49] |
FANG P, HU JH, CHENG ZG, et al. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: a systematic review of phase Ⅱ trials[J]. PLoS One, 2012, 7( 12): e49717. DOI: 10.1371/journal.pone.0049717. |
| [50] |
BRUIX J, TAKAYAMA T, MAZZAFERRO V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation(STORM): a phase 3, randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2015, 16( 13): 1344- 1354. DOI: 10.1016/S1470-2045(15)00198-9. |
| [51] |
SUN C, LI B, LI L, et al. Effect of Huaier granules on patients with middle and advanced liver cancer[J]. J Changchun Univ Chin Med, 2022, 38( 10): 1130- 1133. DOI: 10.13463/j.cnki.cczyy.2022.10.016. |
| [52] |
WANG Z, REN Z, CHEN Y, et al. Adjuvant transarterial chemoembolization for hbv-related hepatocellular carcinoma after resection: a randomized controlled studyadjuvant TACE for HCC after resection[J]. Clin Cancer Res, 2018, 24( 9): 2074- 2081. DOI: 10.1158/1078-0432.CCR-17-2899. |
| [53] |
CHEN Q, SHU C, LAURENCE AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial[J]. Gut, 2018, 67( 11): 2006- 2016. DOI: 10.1136/gutjnl-2018-315983. |
| [54] |
FENG M, TANG C, FENG W, et al. Hepatic artery-infusion chemotherapy improved survival of hepatocellular carcinoma after radical hepatectomy[J]. Onco Targets Ther, 2017, 10: 3001- 3005. DOI: 10.2147/OTT.S136806. |
| [55] |
|



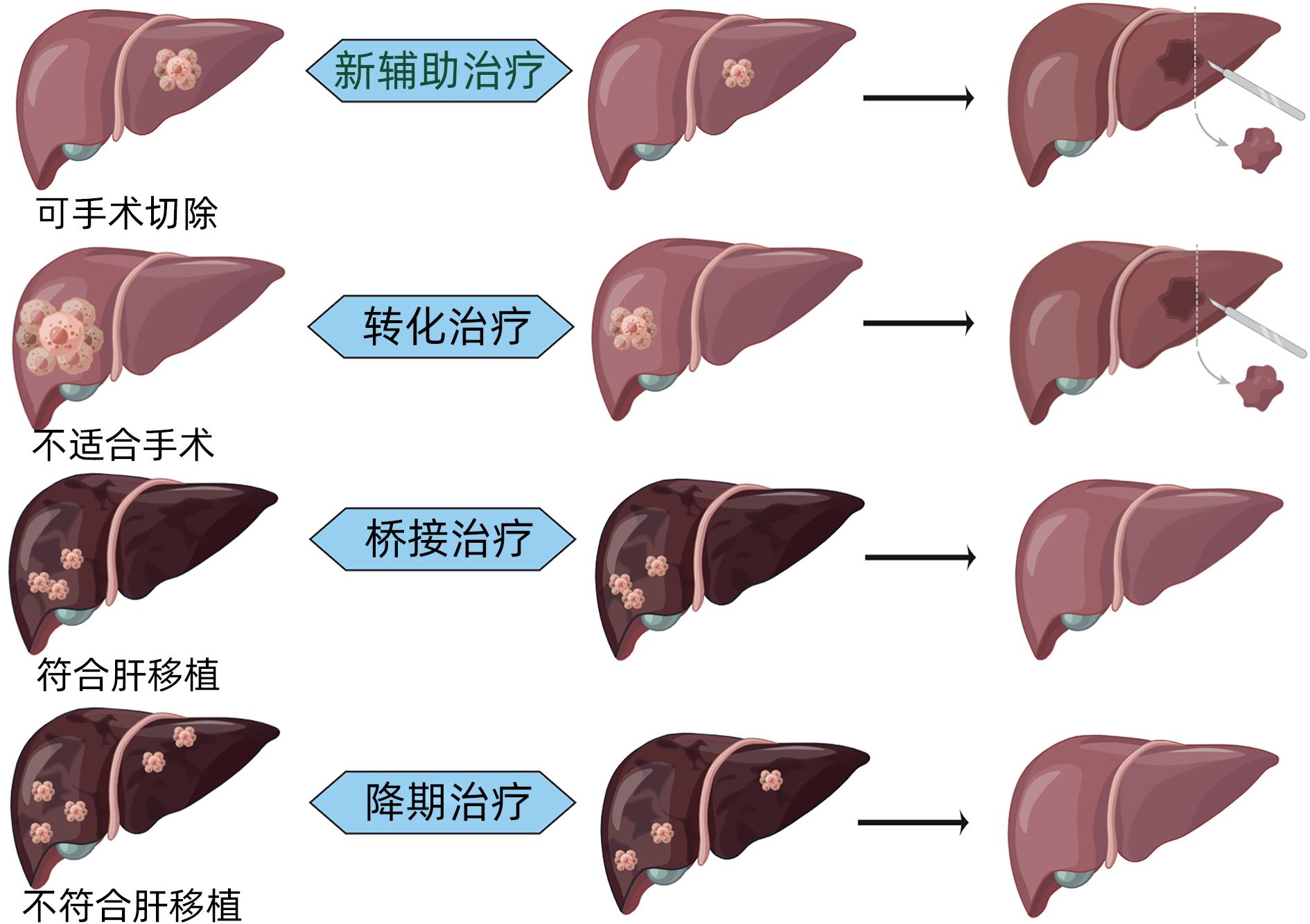




 DownLoad:
DownLoad: