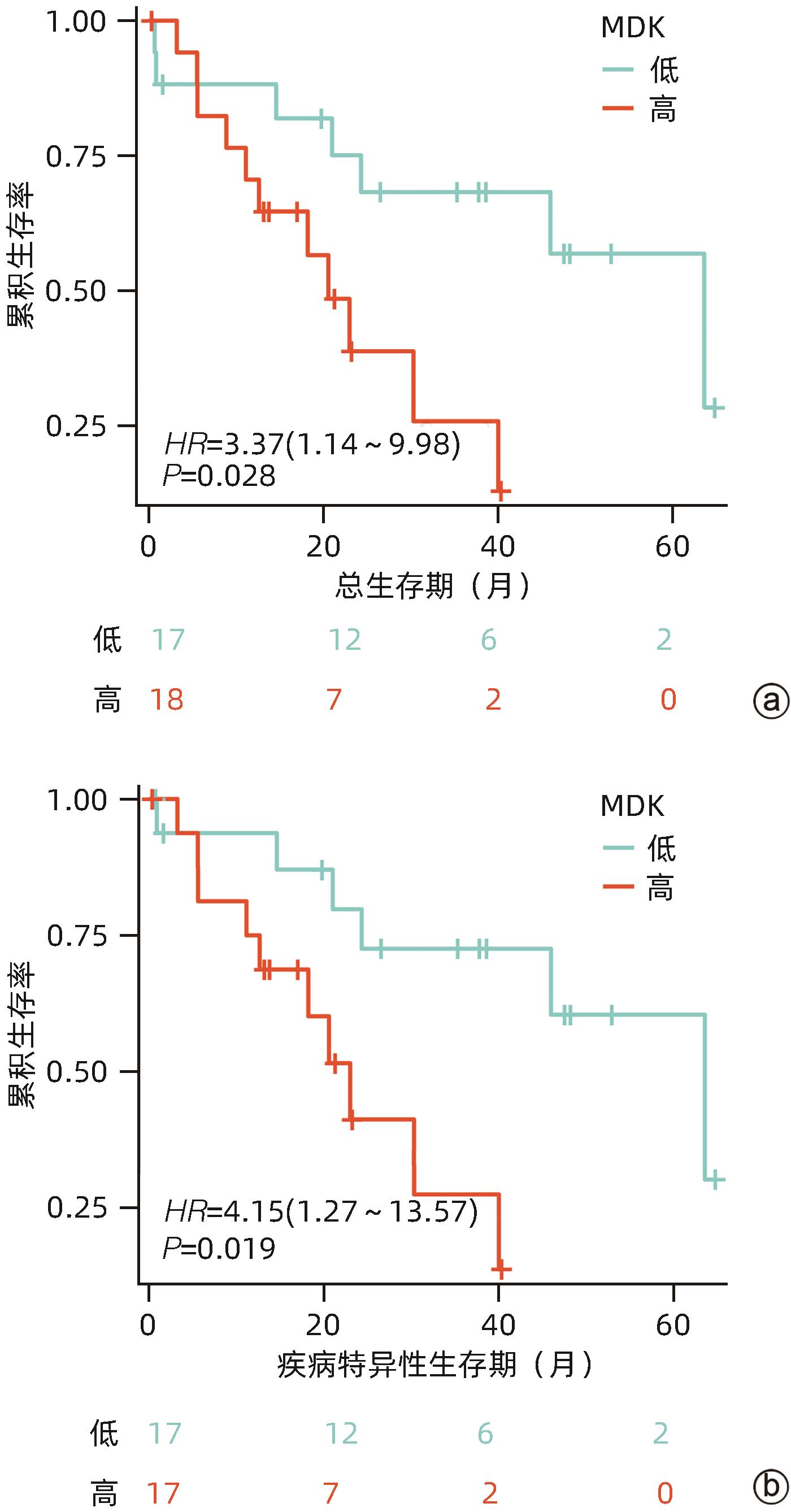
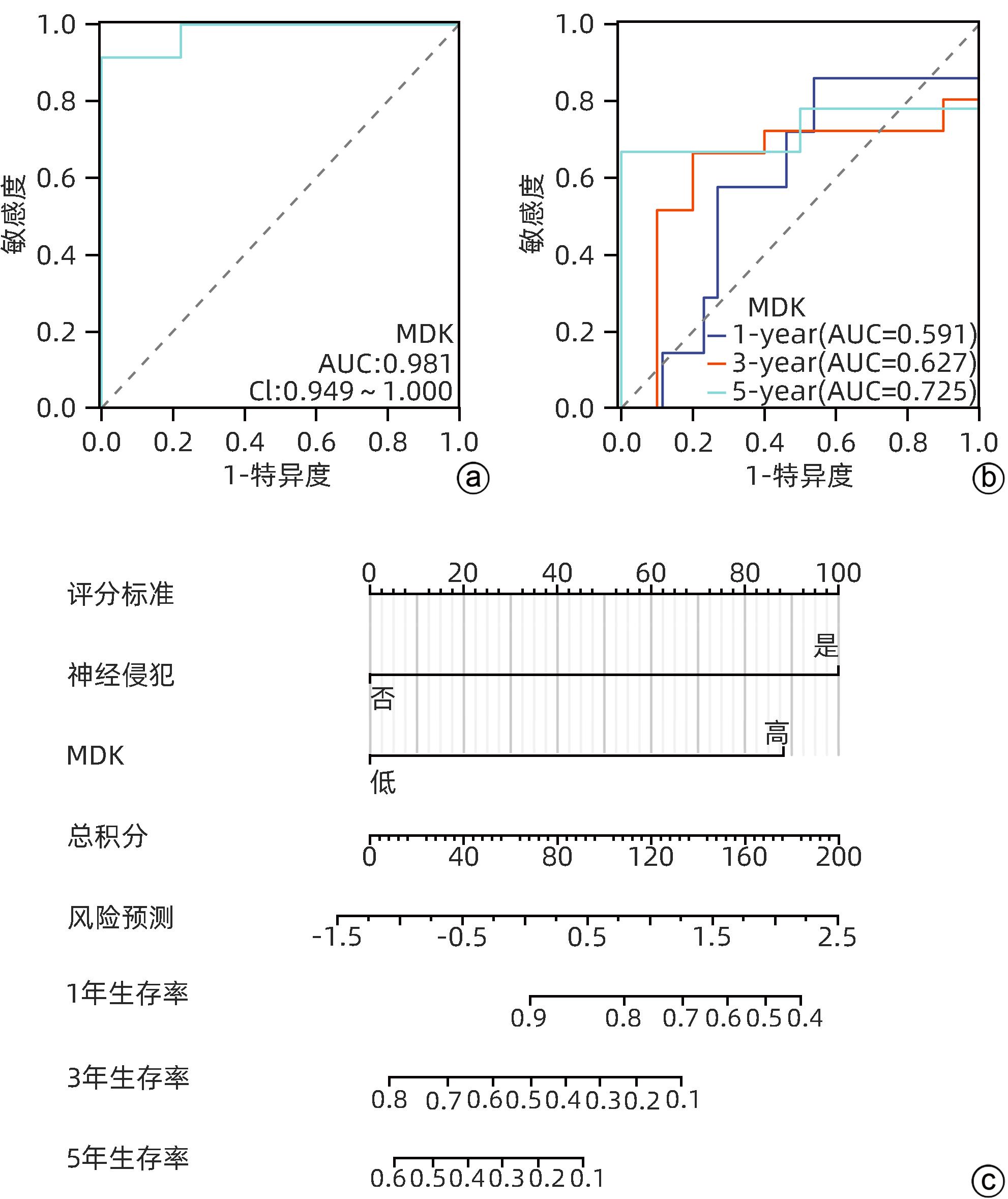
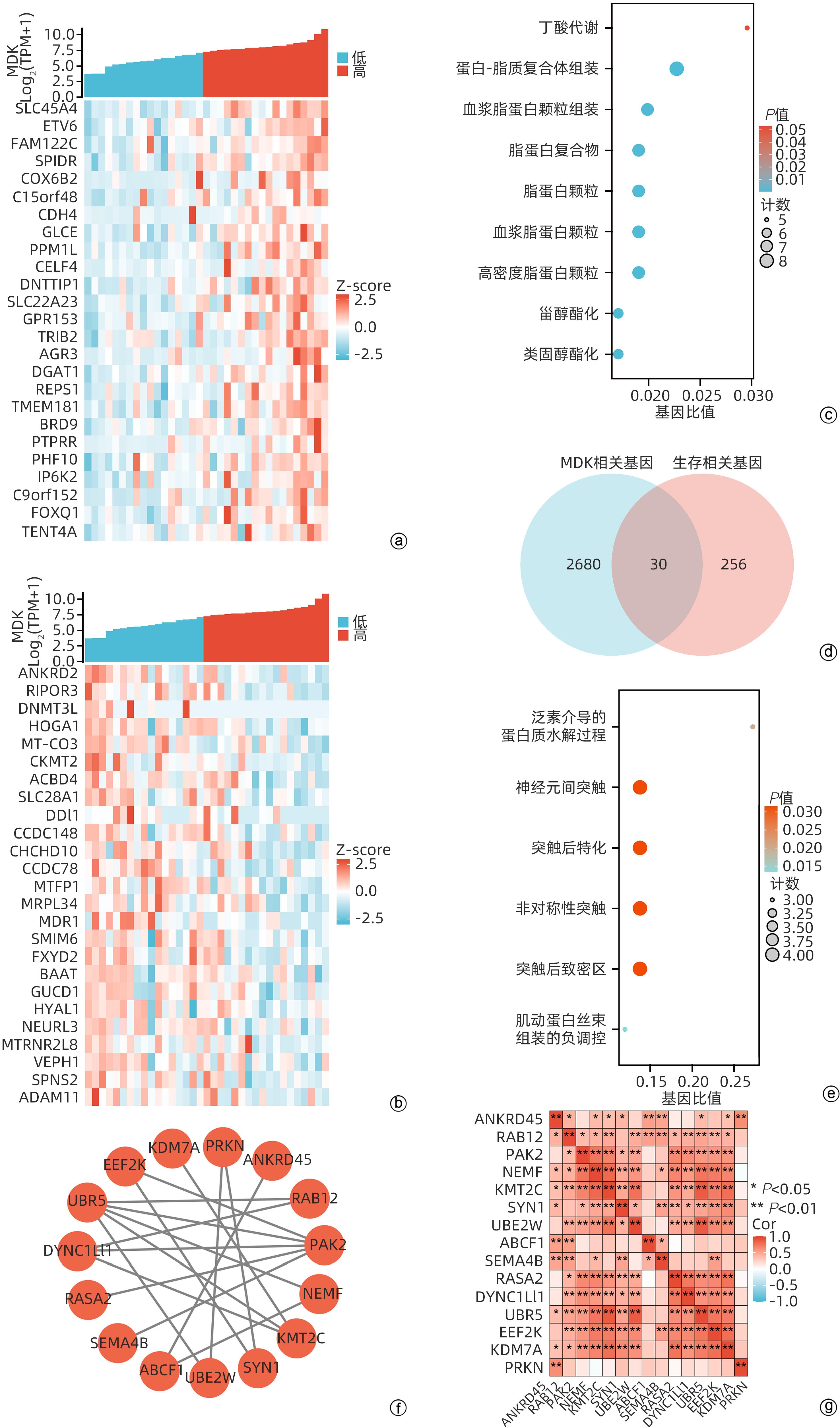
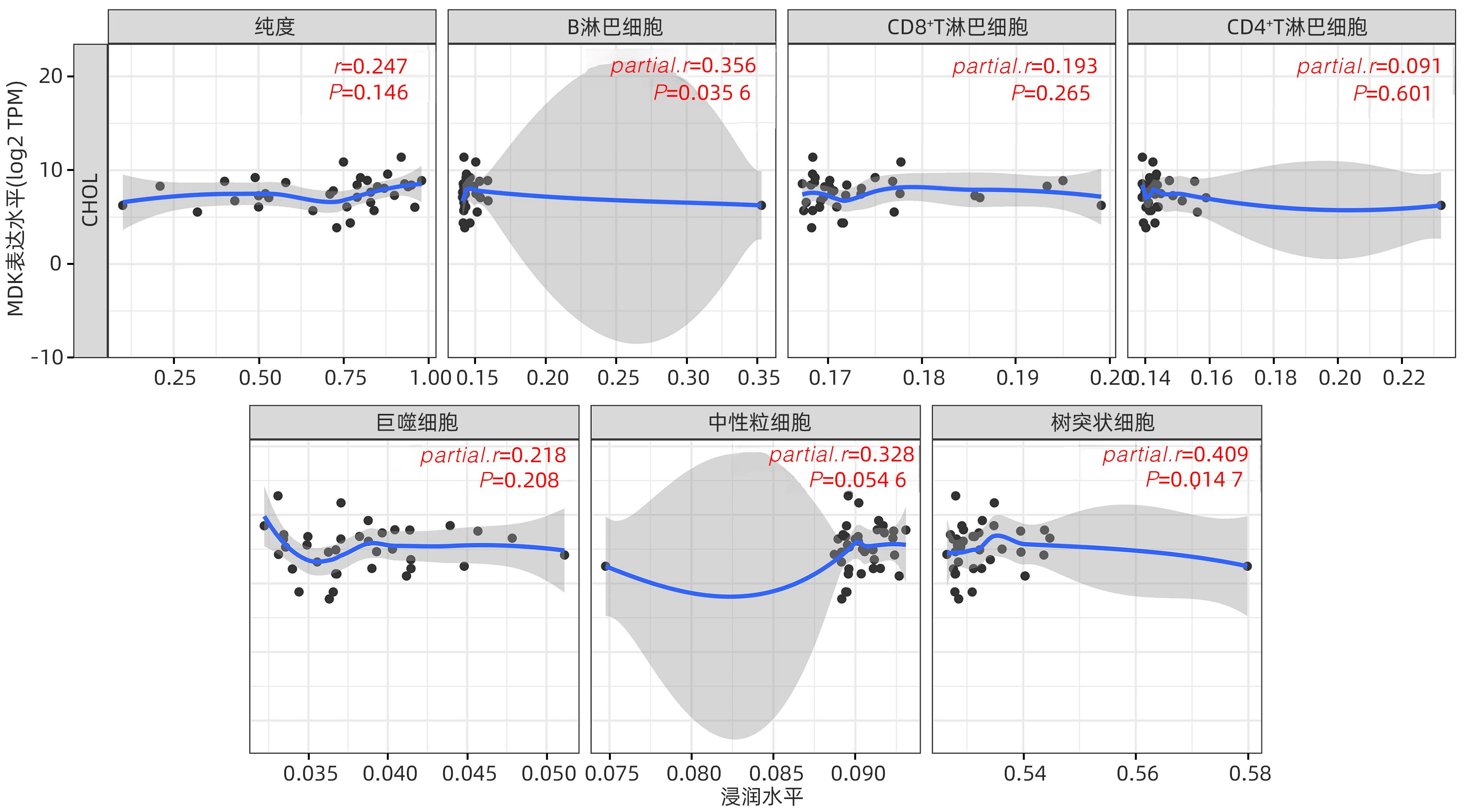
| [1] |
GRETEN TF, SCHWABE R, BARDEESY N, et al. Immunology and immunotherapy of cholangiocarcinoma[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 6): 349- 365. DOI: 10.1038/s41575-022-00741-4. |
| [2] |
BANALES JM, MARIN JJG, LAMARCA A, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. |
| [3] |
|
| [4] |
NAGINO M, HIRANO S, YOSHITOMI H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition[J]. J Hepatobiliary Pancreat Sci, 2021, 28( 1): 26- 54. DOI: 10.1002/jhbp.870. |
| [5] |
LI Y, LI DJ, CHEN J, et al. Application of joint detection of AFP, CA19-9, CA125 and CEA in identification and diagnosis of cholangiocarcinoma[J]. Asian Pac J Cancer Prev, 2015, 16( 8): 3451- 3455. DOI: 10.7314/apjcp.2015.16.8.3451. |
| [6] |
|
| [7] |
ZHANG ZZ, WANG G, YIN SH, et al. Midkine: A multifaceted driver of atherosclerosis[J]. Clin Chim Acta, 2021, 521: 251- 257. DOI: 10.1016/j.cca.2021.07.024. |
| [8] |
CAMPBELL VK, GATELY RP, KRISHNASAMY R, et al. Midkine and chronic kidney disease-associated multisystem organ dysfunctions[J]. Nephrol Dial Transplant, 2021, 36( 9): 1577- 1584. DOI: 10.1093/ndt/gfaa084. |
| [9] |
FILIPPOU PS, KARAGIANNIS GS, CONSTANTINIDOU A. Midkine(MDK) growth factor: A key player in cancer progression and a promising therapeutic target[J]. Oncogene, 2020, 39( 10): 2040- 2054. DOI: 10.1038/s41388-019-1124-8. |
| [10] |
CHOI YW, KIM YH, LEE J, et al. Strong immunoexpression of midkine is associated with multiple lymph node metastases in BRAFV600E papillary thyroid carcinoma[J]. Hum Pathol, 2015, 46( 10): 1557- 1565. DOI: 10.1016/j.humpath.2015.06.018. |
| [11] |
GÜNGÖR C, ZANDER H, EFFENBERGER KE, et al. Notch signaling activated by replication stress-induced expression of midkine drives epithelial-mesenchymal transition and chemoresistance in pancreatic cancer[J]. Cancer Res, 2011, 71( 14): 5009- 5019. DOI: 10.1158/0008-5472.CAN-11-0036. |
| [12] |
YAO X, WANG X, WANG ZS, et al. Clinicopathological and prognostic significance of epithelial mesenchymal transition-related protein expression in intrahepatic cholangiocarcinoma[J]. Onco Targets Ther, 2012, 5: 255- 261. DOI: 10.2147/OTT.S36213. |
| [13] |
ZHANG YJ, ZUO CM, LIU LG, et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer[J]. J Hepatol, 2021, 75( 5): 1128- 1141. DOI: 10.1016/j.jhep.2021.06.023. |
| [14] |
WANG D, BU F, ZHANG WW. The role of ubiquitination in regulating embryonic stem cell maintenance and cancer development[J]. Int J Mol Sci, 2019, 20( 11): 2667. DOI: 10.3390/ijms20112667. |
| [15] |
CHEN Y, XU X, WANG YR, et al. Hypoxia-induced SKA3 promoted cholangiocarcinoma progression and chemoresistance by enhancing fatty acid synthesis via the regulation of PAR-dependent HIF-1a deubiquitylation[J]. J Exp Clin Cancer Res, 2023, 42( 1): 265. DOI: 10.1186/s13046-023-02842-7. |
| [16] |
CEREZO-WALLIS D, CONTRERAS-ALCALDE M, TROULÉ K, et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state[J]. Nat Med, 2020, 26( 12): 1865- 1877. DOI: 10.1038/s41591-020-1073-3. |
| [17] |
ZHAO SL, WANG HJ, NIE YZ, et al. Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity[J]. Cancer Immunol Immunother, 2012, 61( 10): 1745- 1753. DOI: 10.1007/s00262-012-1235-3. |
| [18] |
GUO XF, PAN Y, XIONG M, et al. Midkine activation of CD8 + T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth[J]. Nat Commun, 2020, 11( 1): 2177. DOI: 10.1038/s41467-020-15770-3. |
| [19] |
FLORES-BORJA F, BLAIR P. Mechanisms of induction of regulatory B cells in the tumour microenvironment and their contribution to immunosuppression and pro-tumour responses[J]. Clin Exp Immunol, 2022, 209( 1): 33- 45. DOI: 10.1093/cei/uxac029. |
| [20] |
SHANG J, ZHA HR, SUN YF. Phenotypes, functions, and clinical relevance of regulatory B cells in cancer[J]. Front Immunol, 2020, 11: 582657. DOI: 10.3389/fimmu.2020.582657. |
| [21] |
MICHAUD D, STEWARD CR, MIRLEKAR B, et al. Regulatory B cells in cancer[J]. Immunol Rev, 2021, 299( 1): 74- 92. DOI: 10.1111/imr.12939. |
| [22] |
KATOPODI T, PETANIDIS S, CHARALAMPIDIS C, et al. Tumor-infiltrating dendritic cells: Decisive roles in cancer immunosurveillance, immunoediting, and tumor T cell tolerance[J]. Cells, 2022, 11( 20): 3183. DOI: 10.3390/cells11203183. |
| [23] |
MOLLICA POETA V, MASSARA M, CAPUCETTI A, et al. Chemokines and chemokine receptors: New targets for cancer immunotherapy[J]. Front Immunol, 2019, 10: 379. DOI: 10.3389/fimmu.2019.00379. |
| [24] |
KORBECKI J, BAJDAK-RUSINEK K, KUPNICKA P, et al. The role of CXCL16 in the pathogenesis of cancer and other diseases[J]. Int J Mol Sci, 2021, 22( 7): 3490. DOI: 10.3390/ijms22073490. |
| [25] |
MEZZAPELLE R, LEO M, CAPRIOGLIO F, et al. CXCR4/CXCL12 activities in the tumor microenvironment and implications for tumor immunotherapy[J]. Cancers, 2022, 14( 9): 2314. DOI: 10.3390/cancers14092314. |
| [26] |
SILIŅA K, SOLTERMANN A, ATTAR FM, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma[J]. Cancer Res, 2018, 78( 5): 1308- 1320. DOI: 10.1158/0008-5472.CAN-17-1987. |
| [27] |
XIE N, CAI JB, ZHANG L, et al. Upregulation of B7-H4 promotes tumor progression of intrahepatic cholangiocarcinoma[J]. Cell Death Dis, 2017, 8( 12): 3205. DOI: 10.1038/s41419-017-0015-6. |
| [28] |
KAMIYA T, OHTANI N. The role of immune cells in the liver tumor microenvironment: An involvement of gut microbiota-derived factors[J]. Int Immunol, 2022, 34( 9): 467- 474. DOI: 10.1093/intimm/dxac020. |
| [29] |
QU P, HUANG XJ, ZHOU XC, et al. Loss of CD155 expression predicts poor prognosis in hepatocellular carcinoma[J]. Histopathology, 2015, 66( 5): 706- 714. DOI: 10.1111/his.12584. |
| [30] |
DU XN, ALMEIDA PD, MANIERI N, et al. CD226 regulates natural killer cell antitumor responses via phosphorylation-mediated inactivation of transcription factor FOXO1[J]. Proc Natl Acad Sci U S A, 2018, 115( 50): E11731- E11740. DOI: 10.1073/pnas.1814052115. |







 DownLoad:
DownLoad: