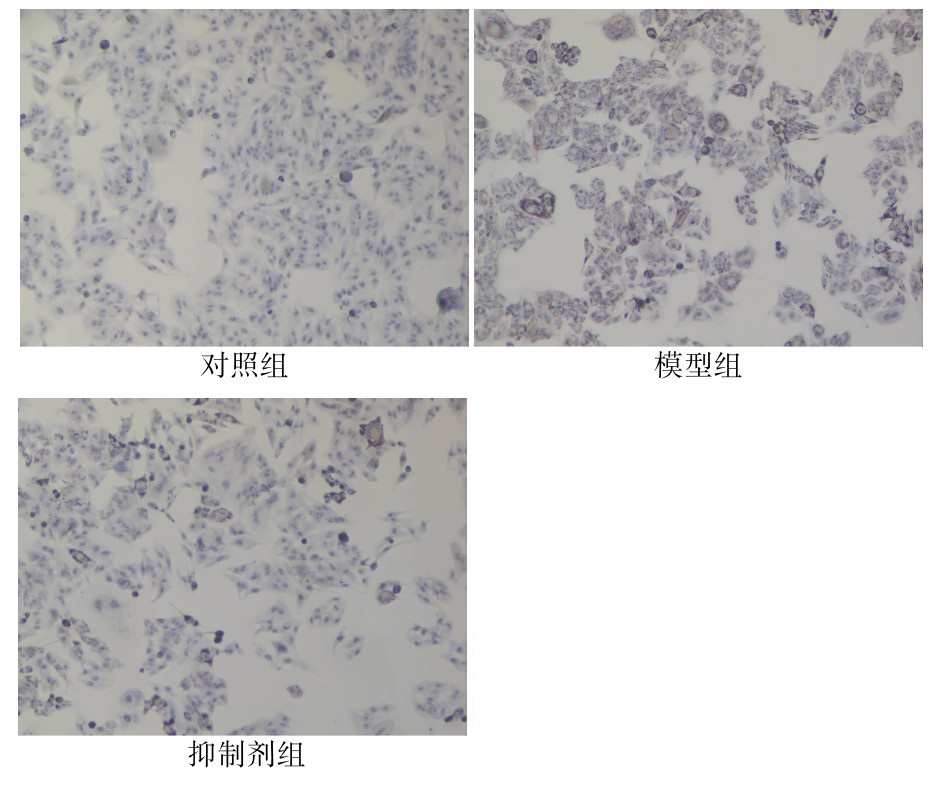
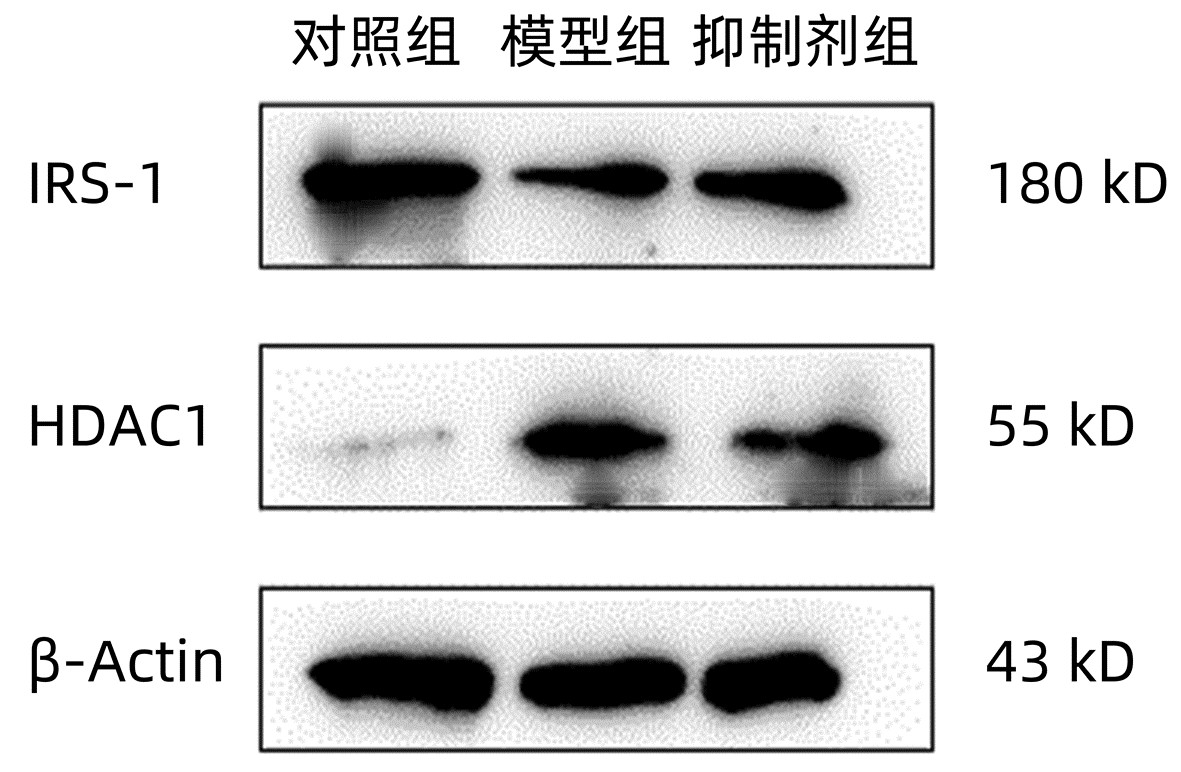
| [1] |
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. |
| [2] |
WANG YH, GAO Y. Research progress in diagnosis and treatment of non-alcoholic fatty liver disease combinated with type 2 diabetes mellitus[J]. J Jilin Univ(Med Edit), 2020, 286(6): 1324-1331. DOI: 10.13481/j.1671-587x.20200634. |
| [3] |
|
| [4] |
|
| [5] |
CASTELLANO D, RAMOS B, OLIVA W, et al. Genome profiling of H3k4me3 histone modification in human adipose tissue during obesity and insulin resistance[J]. Biomedicines, 2021, 9(10): 1363. DOI: 10.3390/biomedicines9101363. |
| [6] |
FU S, YU M, TAN Y, et al. Role of histone deacetylase on nonalcoholic fatty liver disease[J]. Expert Rev Gastroenterol Hepatol, 2021, 15(4): 353-361. DOI: 10.1080/17474124.2021.1854089. |
| [7] |
SODUM N, KUMAR G, BOJJA SL, et al. Epigenetics in NAFLD/NASH: Targets and therapy[J]. Pharmacol Res, 2021, 167: 105484. DOI: 10.1016/j.phrs.2021.105484. |
| [8] |
|
| [9] |
RAJAN PK, UDOH UA, SANABRIA JD, et al. The role of histone acetylation-/methylation-mediated apoptotic gene regulation in hepatocellular carcinoma[J]. Int J Mol Sci, 2020, 21(23): 8894. DOI: 10.3390/ijms21238894. |
| [10] |
JAMES DE, STÖCKLI J, BIRNBAUM MJ. The aetiology and molecular landscape of insulin resistance[J]. Nat Rev Mol Cell Biol, 2021, 22(11): 751-771. DOI: 10.1038/s41580-021-00390-6. |
| [11] |
YANG Q, VIJAYAKUMAR A, KAHN BB. Metabolites as regulators of insulin sensitivity and metabolism[J]. Nat Rev Mol Cell Biol, 2018, 19(10): 654-672. DOI: 10.1038/s41580-018-0044-8. |
| [12] |
MAUDE H, SANCHEZ-CABANILLAS C, CEBOLA I. Epigenetics of hepatic insulin resistance[J]. Front Endocrinol (Lausanne), 2021, 12: 681356. DOI: 10.3389/fendo.2021.681356. |
| [13] |
KHAN S, KOMARYA SK, JENA G. Phenylbutyrate and β-cell function: contribution of histone deacetylases and ER stress inhibition[J]. Epigenomics, 2017, 9(5): 711-720. DOI: 10.2217/epi-2016-0160. |
| [14] |
KHAN S, KUMAR S, JENA G. Valproic acid reduces insulin-resistance, fat deposition and FOXO1-mediated gluconeogenesis in type-2 diabetic rat[J]. Biochimie, 2016, 125: 42-52. DOI: 10.1016/j.biochi.2016.02.014. |










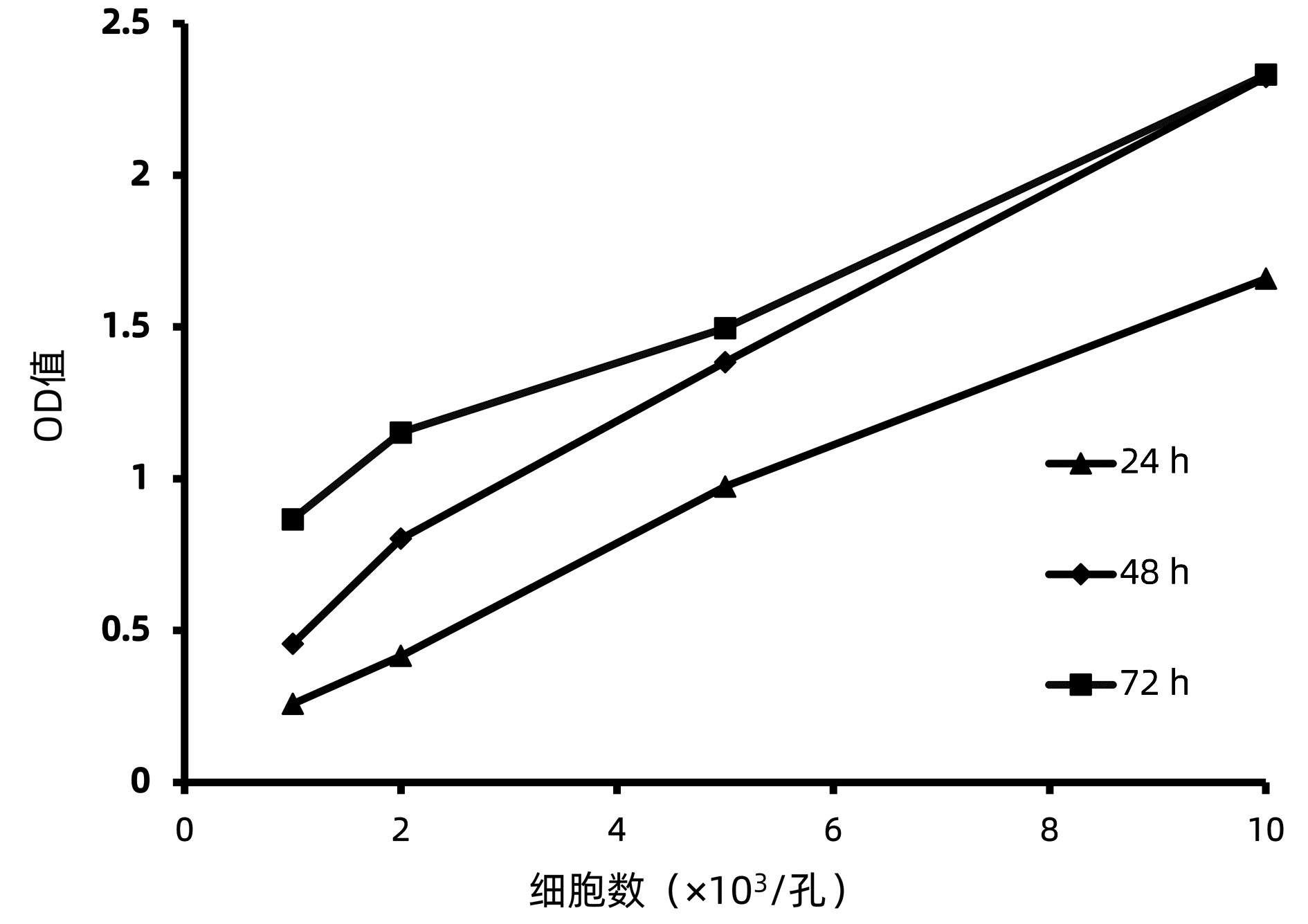




 DownLoad:
DownLoad: