| [1] |
VARGAS J, HAMASAKI M, KAWABATA T, et al. The mechanisms and roles of selective autophagy in mammals[J]. Nat Rev Mol Cell Biol, 2023, 24( 3): 167- 185. DOI: 10.1038/s41580-022-00542-2. |
| [2] |
REN F, ZHANG L, ZHANG X, et al. Inhibition of glycogen synthase kinase 3β promotes autophagy to protect mice from acute liver failure mediated by peroxisome proliferator-activated receptor α[J]. Cell Death Dis, 2016, 7( 3): e2151. DOI: 10.1038/cddis.2016.56. |
| [3] |
XUE R, YANG J, JIA L, et al. Mitofusin2, as a protective target in the liver, controls the balance of apoptosis and autophagy in acute-on-chronic liver failure[J]. Front Pharmacol, 2019, 10: 601. DOI: 10.3389/fphar.2019.00601. |
| [4] |
WU Y, HE Y, WANG F, et al. Lipopolysaccharide inhibits autophagy and promotes inflammatory responses via p38 MAPK-induced proteasomal degradation of Atg13 in hepatic stellate cells[J]. Mediators Inflamm, 2022, 2022: 9603989. DOI: 10.1155/2022/9603989. |
| [5] |
TANG L, WANG X, ZHAO R, et al. Yi-Qi-Jian-Pi formula ameliorates immune function in acute-on-chronic liver failure by upregulating autophagy and mitochondrial biogenesis in CD8(+) T lymphocytes[J]. J Ethnopharmacol, 2023, 308: 116276. DOI: 10.1016/j.jep.2023.116276. |
| [6] |
STAVROPOULOS A, DIVOLIS G, MANIOUDAKI M, et al. Coordinated activation of TGF-β and BMP pathways promotes autophagy and limits liver injury after acetaminophen intoxication[J]. Sci Signal, 2022, 15( 740): eabn4395. DOI: 10.1126/scisignal.abn4395. |
| [7] |
NI HM, BOCKUS A, BOGGESS N, et al. Activation of autophagy protects against acetaminophen-induced hepatotoxicity[J]. Hepatology, 2012, 55( 1): 222- 232. DOI: 10.1002/hep.24690. |
| [8] |
HE YM, SHEN XL, GUO YN, et al. Yinhuang oral liquid protects acetaminophen-induced acute liver injury by regulating the activation of autophagy and Nrf2 signaling[J]. Ecotoxicol Environ Saf, 2022, 244: 114073. DOI: 10.1016/j.ecoenv.2022.114073. |
| [9] |
FAN Z, LI Y, CHEN S, et al. Magnesium isoglycyrrhizinate ameliorates concanavalin A-induced liver injury by inhibiting autophagy[J]. Front Pharmacol, 2022, 12: 794319. DOI: 10.3389/fphar.2021.794319. |
| [10] |
JIA Y, MA L, WANG Y, et al. NLRP3 inflammasome and related cytokines reflect the immune status of patients with HBV-ACLF[J]. Mol Immunol, 2020, 120: 179- 186. DOI: 10.1016/j.molimm.2020.01.011. |
| [11] |
HAN M, LI S, LI L. Verapamil inhibits early acute liver failure through suppressing the NLRP3 inflammasome pathway[J]. J Cell Mol Med, 2021, 25( 13): 5963- 5975. DOI: 10.1111/jcmm.16357. |
| [12] |
WANG Y, CHEN Q, JIAO F, et al. Histone deacetylase 2 regulates ULK1 mediated pyroptosis during acute liver failure by the K68 acetylation site[J]. Cell Death Dis, 2021, 12( 1): 55. DOI: 10.1038/s41419-020-03317-9. |
| [13] |
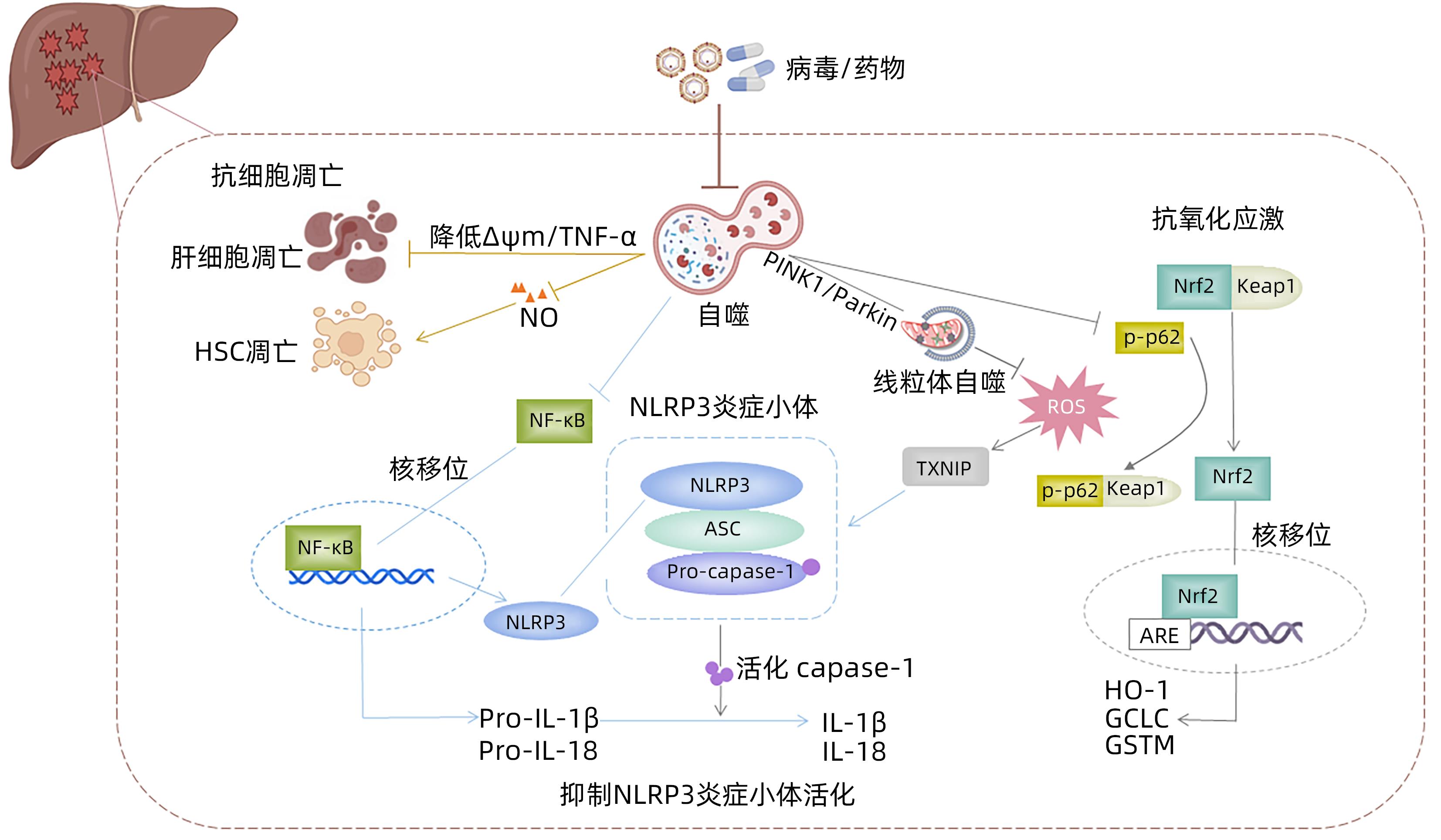
SHAN S, SHEN Z, ZHANG C, et al. Mitophagy protects against acetaminophen-induced acute liver injury in mice through inhibiting NLRP3 inflammasome activation[J]. Biochem Pharmacol, 2019, 169: 113643. DOI: 10.1016/j.bcp.2019.113643. |
| [14] |
HAN J, BAE J, CHOI CY, et al. Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice[J]. Autophagy, 2016, 12( 12): 2326- 2343. DOI: 10.1080/15548627.2016.1235124. |
| [15] |
BIASIZZO M, KOPITAR-JERALA N. Interplay between NLRP3 inflammasome and autophagy[J]. Front Immunol, 2020, 11: 591803. DOI: 10.3389/fimmu.2020.591803. |
| [16] |
WANG T, LU Z, QU XH, et al. Chrysophanol-8-O-glucoside protects mice against acute liver injury by inhibiting autophagy in hepatic stellate cells and inflammatory response in liver-resident macrophages[J]. Front Pharmacol, 2022, 13: 951521. DOI: 10.3389/fphar.2022.951521. |
| [17] |
VARıŞLı B, CAGLAYAN C, KANDEMIR FM, et al. Chrysin mitigates diclofenac-induced hepatotoxicity by modulating oxidative stress, apoptosis, autophagy and endoplasmic reticulum stress in rats[J]. Mol Biol Rep, 2023, 50( 1): 433- 442. DOI: 10.1007/s11033-022-07928-7. |
| [18] |
OAMI T, WATANABE E, HATANO M, et al. Blocking liver autophagy accelerates apoptosis and mitochondrial injury in hepatocytes and reduces time to mortality in a murine sepsis model[J]. Shock, 2018, 50( 4): 427- 434. DOI: 10.1097/SHK.0000000000001040. |
| [19] |
WANG H, NI HM, CHAO X, et al. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice[J]. Redox Biol, 2019, 22: 101148. DOI: 10.1016/j.redox.2019.101148. |
| [20] |
SHEN Z, WANG Y, SU Z, et al. Activation of p62-keap1-Nrf2 antioxidant pathway in the early stage of acetaminophen-induced acute liver injury in mice[J]. Chem Biol Interact, 2018, 282: 22- 28. DOI: 10.1016/j.cbi.2018.01.008. |
| [21] |
RUART M, CHAVARRIA L, CAMPRECIÓS G, et al. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury[J]. J Hepatol, 2019, 70( 3): 458- 469. DOI: 10.1016/j.jhep.2018.10.015. |
| [22] |
YANG J, LI J, GUO H, et al. An experimental study reveals the protective effect of autophagy against realgar-induced liver injury via suppressing ROS-mediated NLRP3 inflammasome pathway[J]. Int J Mol Sci, 2022, 23( 10): 5697. DOI: 10.3390/ijms23105697. |
| [23] |
WANG Q, JIA F, GUO C, et al. PINK1/Parkin-mediated mitophagy as a protective mechanism against AFB(1)-induced liver injury in mice[J]. Food Chem Toxicol, 2022, 164: 113043. DOI: 10.1016/j.fct.2022.113043. |
| [24] |
AHMEDY OA, SALEM HH, SAYED NH, et al. Naringenin affords protection against lipopolysaccharide/D-galactosamine-induced acute liver failure: Role of autophagy[J]. Arch Biochem Biophys, 2022, 717: 109121. DOI: 10.1016/j.abb.2022.109121. |
| [25] |
CHEN Q, WANG Y, JIAO FZ, et al. Histone deacetylase 6 inhibitor ACY1215 offers a protective effect through the autophagy pathway in acute liver failure[J]. Life Sci, 2019, 238: 116976. DOI: 10.1016/j.lfs.2019.116976. |
| [26] |
TIAN Z, CHEN Y, YAO N, et al. Role of mitophagy regulation by ROS in hepatic stellate cells during acute liver failure[J]. Am J Physiol Gastrointest Liver Physiol, 2018, 315( 3): G374-G384. DOI: 10.1152/ajpgi.00032.2018. |
| [27] |
JIA Y, LI Y, WANG YJ. Research progress on exosome targeted delivery of nucleic acid molecules[J]. China Med Herald, 2023, 20( 3): 33- 36, 49. DOI: 10.20047/j.issn1673-7210.2023.03.07. |
| [28] |
|
| [29] |
COLLETTI M, CEGLIE D, DI GIANNATALE A, et al. Autophagy and exosomes relationship in cancer: friends or foes?[J]. Front Cell Dev Biol, 2021, 8: 614178. DOI: 10.3389/fcell.2020.614178. |
| [30] |
SHEN Y, MALIK SA, AMIR M, et al. Decreased hepatocyte autophagy leads to synergistic IL-1β and TNF mouse liver injury and inflammation[J]. Hepatology, 2020, 72( 2): 595- 608. DOI: 10.1002/hep.31209. |
| [31] |
JIAO Y, ZHANG Y, SHI HL, et al. A bioinformatics analysis of differentially expressed proteins in plasma exosome of acute-on-chronic liver failure patients with different prognoses[J]. J Clin Hepatol, 2021, 37( 4): 834- 840. DOI: 10.3969/j.issn.1001-5256.2021.04.022. |
| [32] |
GAO S, FAN YC, HAN LY, et al. Serum exosomal long noncoding RNA nuclear-enriched abundant transcript 1 predicts 90-day mortality in acute-on-chronic hepatitis B liver failure[J]. Expert Rev Clin Immunol, 2021, 17( 7): 789- 797. DOI: 10.1080/1744666X.2021.1933442. |
| [33] |
JIAO Y, LU W, XU P, et al. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure[J]. Hepatol Int, 2021, 15( 4): 957- 969. DOI: 10.1007/s12072-021-10217-3. |
| [34] |
YANG B, DUAN W, WEI L, et al. Bone marrow mesenchymal stem cell-derived hepatocyte-like cell exosomes reduce hepatic ischemia/reperfusion injury by enhancing autophagy[J]. Stem Cells Dev, 2020, 29( 6): 372- 379. DOI: 10.1089/scd.2019.0194. |
| [35] |
LIN D, CHEN H, XIONG J, et al. Mesenchymal stem cells exosomal let-7a-5p improve autophagic flux and alleviate liver injury in acute-on-chronic liver failure by promoting nuclear expression of TFEB[J]. Cell Death Dis, 2022, 13( 10): 865. DOI: 10.1038/s41419-022-05303-9. |
| [36] |
LIU YM, MA JH, ZENG QL, et al. MiR-19a affects hepatocyte autophagy via regulating lncRNA NBR2 and AMPK/PPARα in D-GalN/Lipopolysaccharide-stimulated hepatocytes[J]. J Cell Biochem, 2018, 119( 1): 358- 365. DOI: 10.1002/jcb.26188. |
| [37] |
JIANG N, ZHANG J, PING J, et al. Salvianolic acid B inhibits autophagy and activation of hepatic stellate cells induced by TGF-β1 by downregulating the MAPK pathway[J]. Front Pharmacol, 2022, 13: 938856. DOI: 10.3389/fphar.2022.938856. |
| [38] |
GUO E, LI R, YANG J, et al. FK866 attenuates acute hepatic failure through c-jun-N-terminal kinase(JNK)-dependent autophagy[J]. Sci Rep, 2017, 7( 1): 2206. DOI: 10.1038/s41598-017-02318-7. |
| [39] |
YANG Y, YING G, WU F, et al. sTim-3 alleviates liver injury via regulation of the immunity microenvironment and autophagy[J]. Cell Death Discov, 2020, 6: 62. DOI: 10.1038/s41420-020-00299-7. |
| [40] |
WANG Y, WANG JL, MA HC, et al. Mesenchymal stem cells increase heme oxygenase 1-activated autophagy in treatment of acute liver failure[J]. Biochem Biophys Res Commun, 2019, 508( 3): 682- 689. DOI: 10.1016/j.bbrc.2018.11.146. |









 本站查看
本站查看




 DownLoad:
DownLoad: