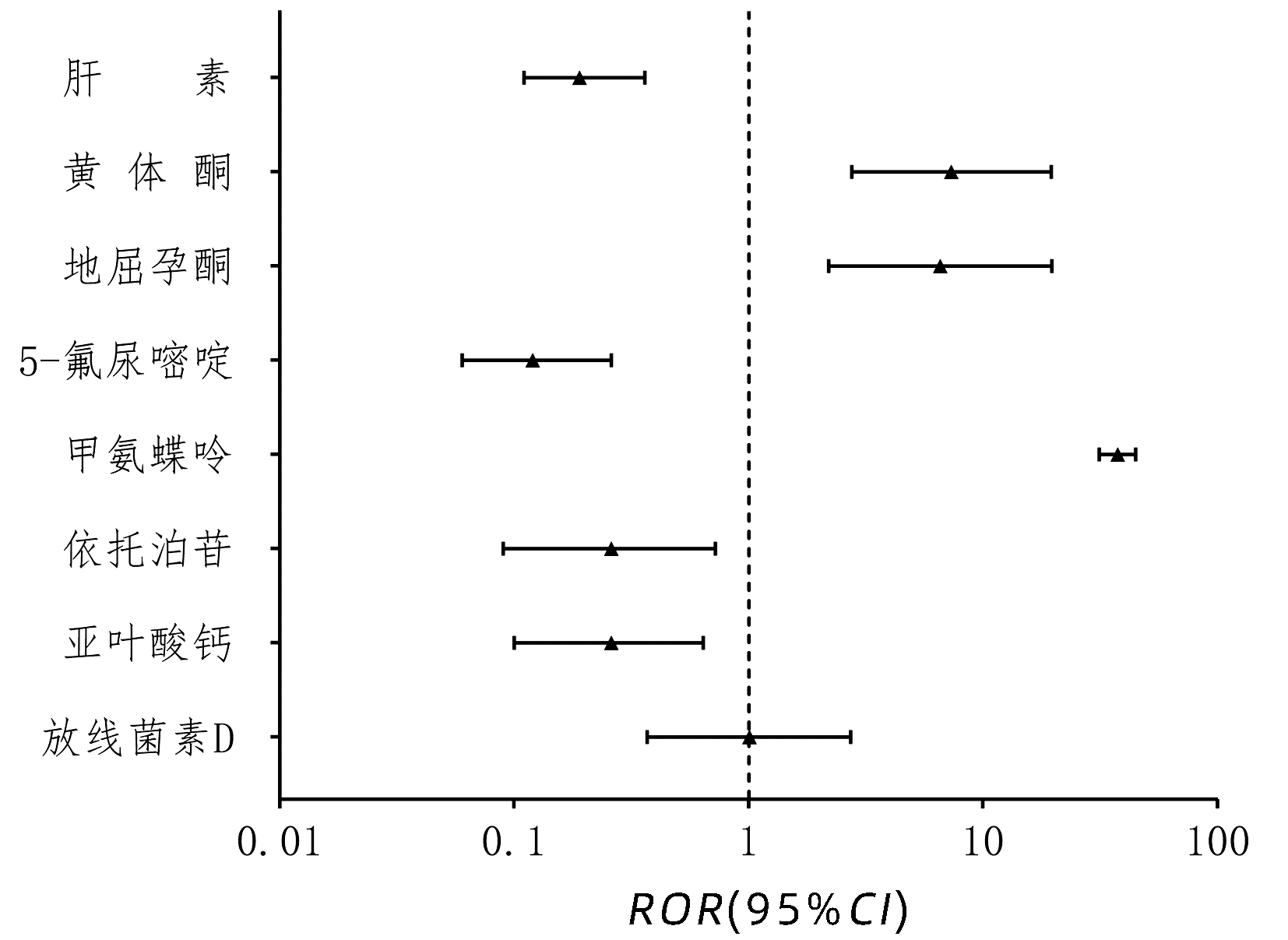
| [1] |
|
| [2] |
ITO S. Mother and child: medication use in pregnancy and lactation[J]. Clin Pharmacol Ther, 2016, 100(1): 8-11. DOI: 10.1002/cpt.383. |
| [3] |
LI L. Effects of puerarin with insulin aspart on biochemical indexes and artery blood flow parameters related to the fetus of pregnant women with Gestational diabetes[J]. World Chi Med, 2018, 13(5): 1134-1138. DOI: 10.3969/j.issn.1673-7202.2018.05.021. |
| [4] |
|
| [5] |
|
| [6] |
HOU Y, YE X, WU G, et al. A comparison of disproportionality analysis methods in national adverse drug reaction databases of China[J]. Expert Opin Drug Saf, 2014, 13(7): 853-857. DOI: 10.1517/14740338.2014.915938. |
| [7] |
van PUIJENBROEK EP, BATE A, LEUFKENS HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions[J]. Pharmacoepidemiol Drug Saf, 2002, 11(1): 3-10. DOI: 10.1002/pds.668. |
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
LI SJ, CHEN L, WU S, et al. Analysis and application of measures of dis-proportionality in methotrexate adverse reaction signal mining[J]. Anti-tumor Pharm, 2018, 8(2): 274-278, 287. DOI: 10.3969/j.issn.2095-1264.2018.02.34. |
| [12] |
|
| [13] |
SONG SQ, ZHANG GN. Combination chemotherapy of blemycin etoposide cisplatin or carboplatin for the treatment of malignant gestational trophoblastic tumor[J]. Chin J Clin Obstet Gynecol, 2006, (4): 248-250. DOI: 10.3969/j.issn.1672-1861.2006.04.003. |
| [14] |
YUAN SW, CEN Y. Diagnosis, treatment and research progress of gestational trophoblastic tumor[J/CD]. J World Latest Med Inf, 2019, 19(48): 324-325. DOI: 10.19613/j.cnki.1671-3141.2019.48.223. |
| [15] |
Gynecological oncology Committee of China Anti Cancer Association. Guidelines for the diagnosis and treatment of gestational trophoblastic diseases (2021 Edition)[J]. China Oncol, 2021, 31(6): 520-532. DOI: 10.19401/j.cnki.1007-3639.2021.06.10. |
| [16] |
WANG LJ, LIN HX, LIN ZQ. Interpretation of 2021 NCCN clinical practice guide for gestational trophoblastic tumor (2nd Edition)[J]. Chin J Pract Gynecol Obstetr, 2021, 37(5): 564-569. DOI: 10.19538/j.fk2021050115. 王丽娟, 林海雪, 林仲秋. 《2021 NCCN妊娠滋养细胞肿瘤临床实践指南(第2版)》解读[J]. 中国实用妇科与产科杂志, 2021, 37(5): 564-569. DOI: 10.19538/j.fk2021050115. |
| [17] |
LIANG Y, MENG Z, CANG HQ, et al. Acute drug-induced liver injury caused by vancomycin hydrochloride for injection: one case report[J]. J Pharm, 2021, 18(8): 789-792. DOI: 10.19803/j.1672-8629.2021.08.20. |
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
WANG Y, YE ZK, CUI XL, et al. Pharmaceutical care of a patient with liver injury induced by omeprazole or octreotide[J]. Chin J Clin Pharmacol, 2017, 33(19): 1959-1960. DOI: 10.13699/j.cnki.1001-6821.2017.19.031. |
| [22] |
KATSUHISA I, HIROAKI Y. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy[J]. Drug Metab Pharmacokinet, 2014, 29(1): 12-19. DOI: 10.2133/dmpk.dmpk-13-rv-119. |
| [23] |
WANG W, ZHOU H, LIU L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review[J]. Eur J Med Chem, 2018, 158: 502-516. DOI: 10.1016/j.ejmech.2018.09.027. |
| [24] |
BAI ZF, GAO Y, ZUO XB, et al. Progress in research on the pathogenesis of immune regulation and idiosyncratic drug-induced liver injury[J]. Acta Pharm Sin, 2017, 52(7): 1019-1026. DOI: 10.16438/j.0513-4870.2017-0315. |
| [25] |
GUO YM, TU C, HE Q, et al. Safety administration strategy for heshouwu (polygoni multiflori radix) based on properties and actions of Chinese medicine[J]. J Tradit Chin Med, 2018, 59(9): 721-724. DOI: 10.13288/j.11-2166/r.2018.09.001. |
| [26] |
TSAKIRIDIS I, GIOULEKA S, MAMOPOULOS A, et al. Diagnosis and management of ectopic pregnancy: a comparative review of major national guidelines[J]. Obstet Gynecol Surv, 2020, 75(10): 611-623. DOI: 10.1097/OGX.0000000000000832. |
| [27] |
PO L, THOMAS J, MILLS K, et al. Guideline No. 414: management of pregnancy of unknown location and tubal and nontubal ectopic pregnancies[J]. J Obstet Gynaecol Can, 2021, 43(5): 614-630. e1. DOI: 10.1016/j.jogc.2021.01.002. |
| [28] |
HU TX, LYU XQ, LI L, et al. Analysis of 4217 cases of adverse drug reactions induced by progesterone oral agents[J]. J Pharm, 2018, 15(12): 728-732. DOI: 10.3969/j.issn.1672-8629.2018.12.005. |








 本站查看
本站查看





 DownLoad:
DownLoad: