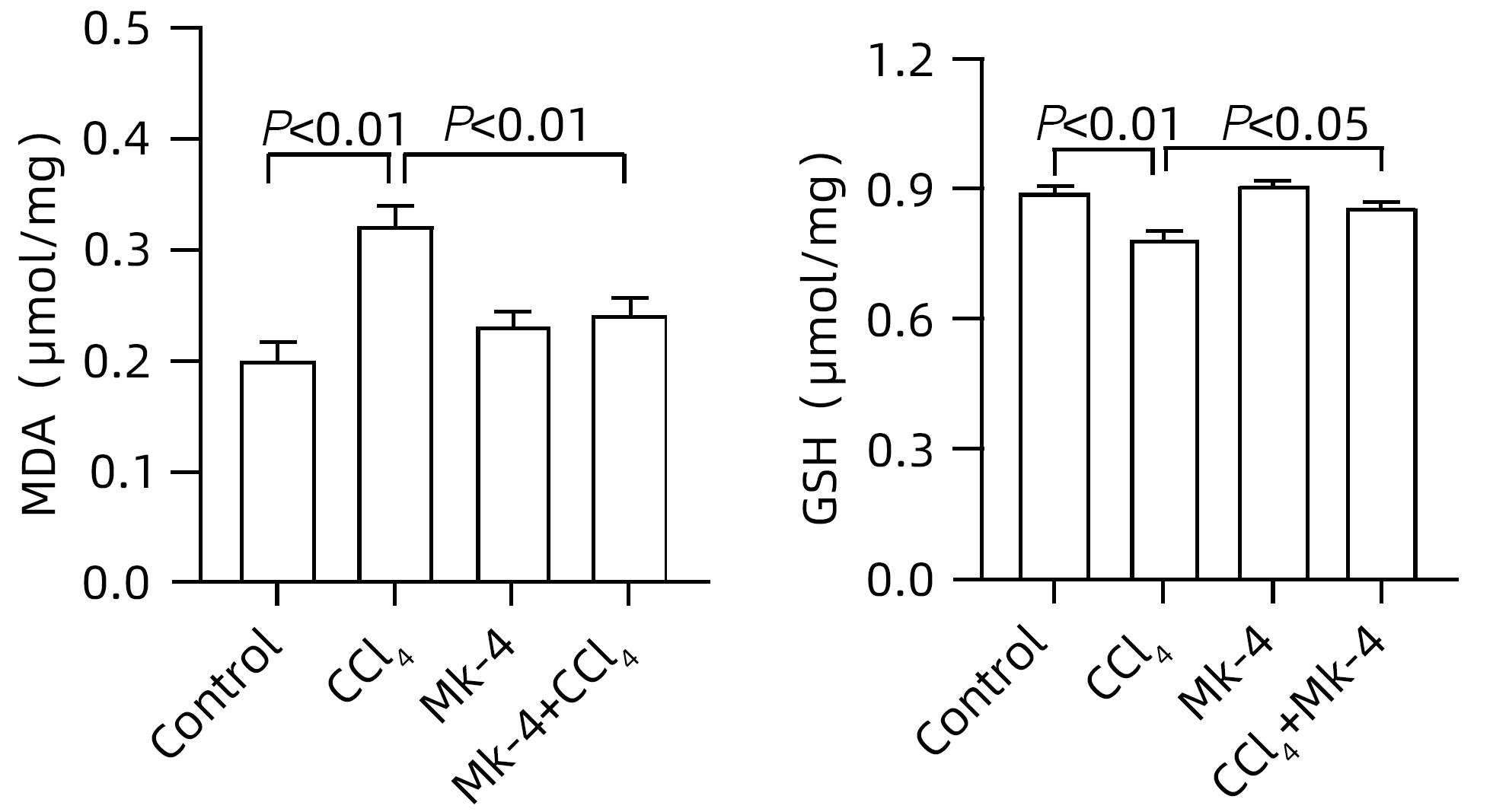
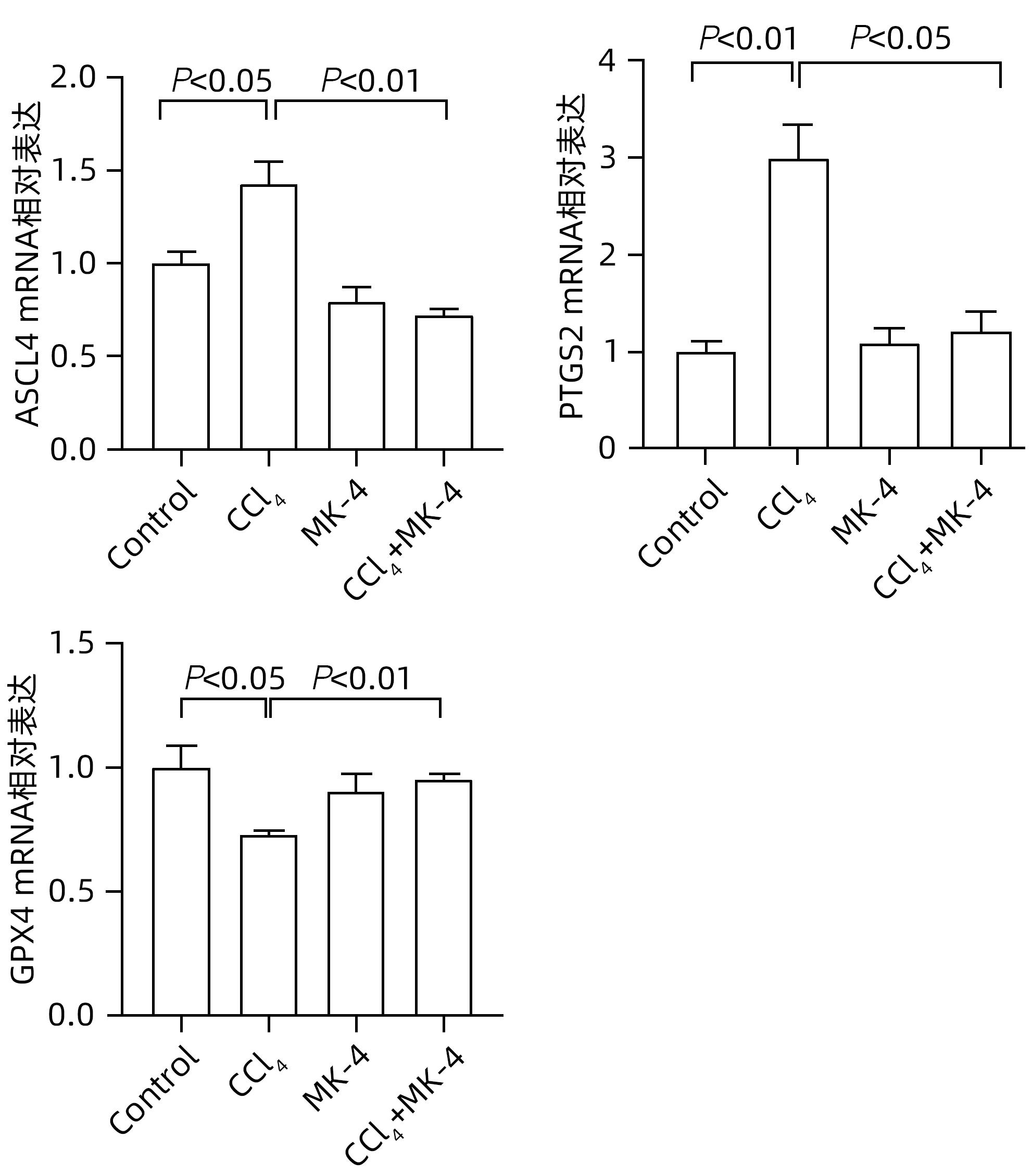
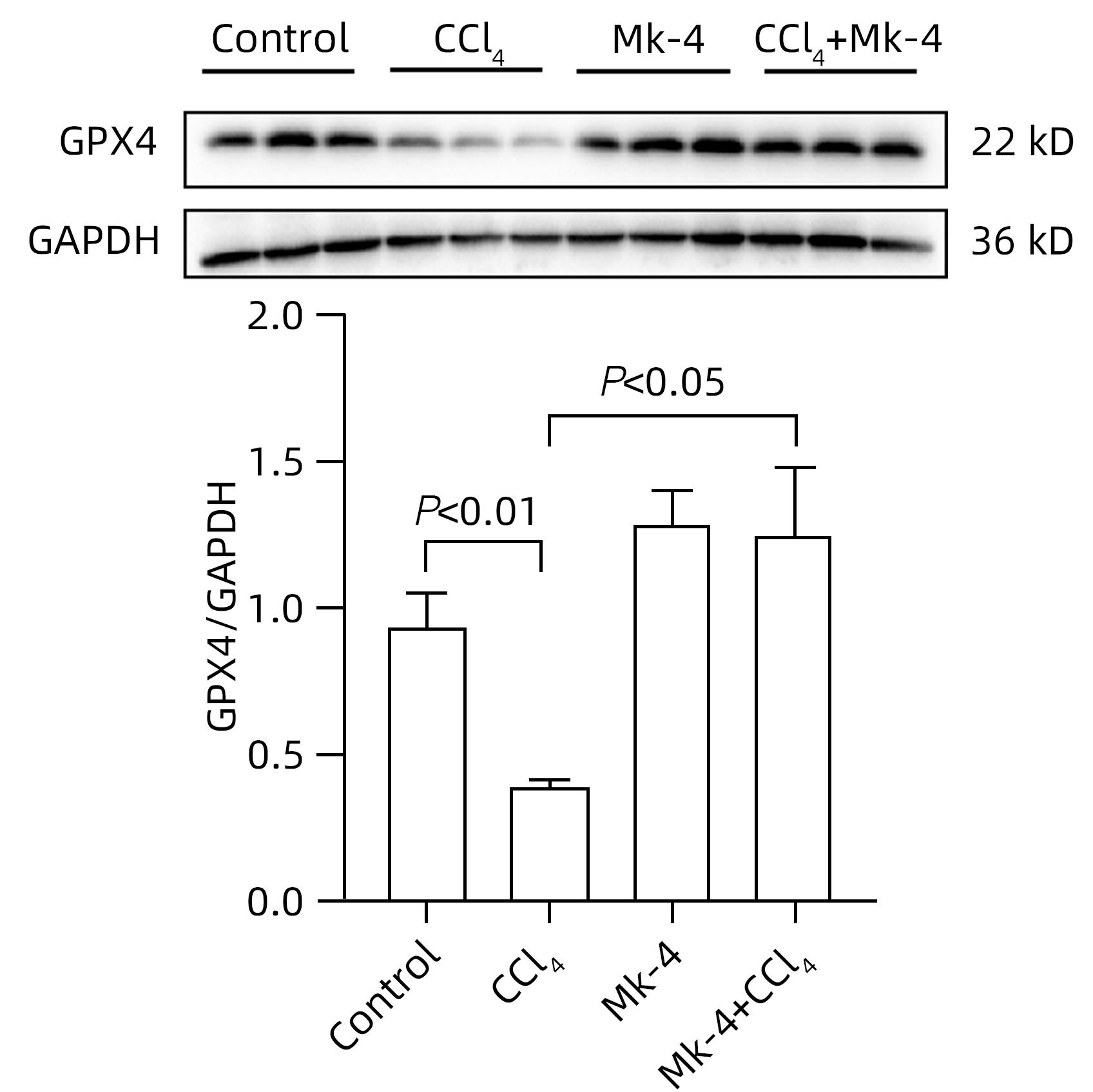
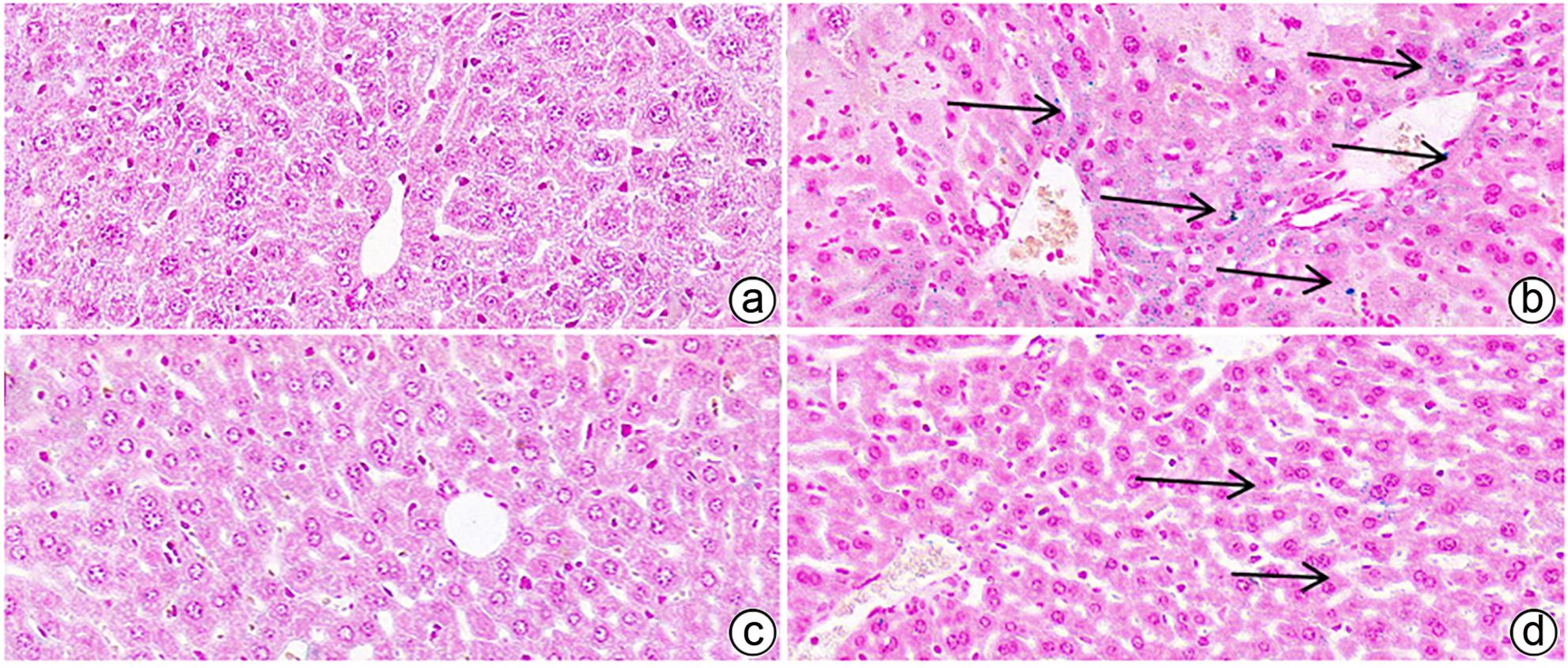
| [1] |
SHRESTHA DB, BUDHATHOKI P, SEDHAI YR, et al. N-acetyl cysteine versus standard of care for non-acetaminophen induced acute liver injury: A systematic review and meta-analysis[J]. Ann Hepatol, 2021, 24: 100340. DOI: 10.1016/j.aohep.2021.100340. |
| [2] |
BHAKUNI GS, BEDI O, BARIWAL J, et al. Animal models of hepatotoxicity[J]. Inflamm Res, 2016, 65( 1): 13- 24. DOI: 10.1007/s00011-015-0883-0. |
| [3] |
LIANG DG, MINIKES AM, JIANG XJ. Ferroptosis at the intersection of lipid metabolism and cellular signaling[J]. Mol Cell, 2022, 82( 12): 2215- 2227. DOI: 10.1016/j.molcel.2022.03.022. |
| [4] |
JIANG XJ, STOCKWELL BR, CONRAD M. Ferroptosis: Mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22( 4): 266- 282. DOI: 10.1038/s41580-020-00324-8. |
| [5] |
LIN FY, CHEN WY, ZHOU JH, et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury[J]. Cell Death Dis, 2022, 13( 3): 271. DOI: 10.1038/s41419-022-04708-w. |
| [6] |
VERVOORT LM, RONDEN JE, THIJSSEN HH. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation[J]. Biochem Pharmacol, 1997, 54( 8): 871- 876. DOI: 10.1016/s0006-2952(97)00254-2. |
| [7] |
LI JR, LIN JC, WANG H, et al. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons[J]. J Neurosci, 2003, 23( 13): 5816- 5826. DOI: 10.1523/JNEUROSCI.23-13-05816.2003. |
| [8] |
MISHIMA E, ITO J, WU ZJ, et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor[J]. Nature, 2022, 608( 7924): 778- 783. DOI: 10.1038/s41586-022-05022-3. |
| [9] |
DIXON SJ, LEMBERG KM, LAMPRECHT MR, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death[J]. Cell, 2012, 149( 5): 1060- 1072. DOI: 10.1016/j.cell.2012.03.042. |
| [10] |
|
| [11] |
SAMRA YA, HAMED MF, EL-SHEAKH AR. Hepatoprotective effect of allicin against acetaminophen-induced liver injury: Role of inflammasome pathway, apoptosis, and liver regeneration[J]. J Biochem Mol Toxicol, 2020, 34( 5): e22470. DOI: 10.1002/jbt.22470. |
| [12] |
SATO T, SCHURGERS LJ, UENISHI K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women[J]. Nutr J, 2012, 11: 93. DOI: 10.1186/1475-2891-11-93. |
| [13] |
YANG WS, SRIRAMARATNAM R, WELSCH ME, et al. Regulation of ferroptotic cancer cell death by GPX4[J]. Cell, 2014, 156( 1-2): 317- 331. DOI: 10.1016/j.cell.2013.12.010. |
| [14] |
STOCKWELL BR, ANGELI JPF, BAYIR H, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease[J]. Cell, 2017, 171( 2): 273- 285. DOI: 10.1016/j.cell.2017.09.021. |
| [15] |
PIETRANGELO A. Genetics, genetic testing, and management of hemochromatosis: 15 years since hepcidin[J]. Gastroenterology, 2015, 149( 5): 1240- 1251.e4. DOI: 10.1053/j.gastro.2015.06.045. |
| [16] |
ZHANG L, LIAO YQ, XIA QC, et al. Ferroptosis regulatory signaling pathway and its research progress in related diseases[J]. Chin J Clin Pharmacol Ther, 2022, 27( 2): 227- 234. DOI: 10.12092/j.issn.1009-2501.2022.02.015. |
| [17] |
YANG WS, STOCKWELL BR. Ferroptosis: Death by lipid peroxidation[J]. Trends Cell Biol, 2016, 26( 3): 165- 176. DOI: 10.1016/j.tcb.2015.10.014. |
| [18] |
FENG H, STOCKWELL BR. Unsolved mysteries: How does lipid peroxidation cause ferroptosis[J]. PLoS Biol, 2018, 16( 5): e2006203. DOI: 10.1371/journal.pbio.2006203. |








 DownLoad:
DownLoad: