| [1] |
LLOVET JM, de BAERE T, KULIK L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 5): 293- 313. DOI: 10.1038/s41575-020-00395-0. |
| [2] |
GHAVIMI S, APFEL T, AZIMI H, et al. Management and treatment of hepatocellular carcinoma with immunotherapy: a review of current and future options[J]. J Clin Transl Hepatol, 2020, 8( 2): 168- 176. DOI: 10.14218/JCTH.2020.00001. |
| [3] |
LIU YE, ZONG J, CHEN XJ, et al. Cryoablation combined with radiotherapy for hepatic malignancy: Five case reports[J]. World J Gastrointest Oncol, 2020, 12( 2): 237- 247. DOI: 10.4251/wjgo.v12.i2.237. |
| [4] |
KIM R, KANG TW, DI CHA, et al. Percutaneous cryoablation for perivascular hepatocellular carcinoma: Therapeutic efficacy and vascular complications[J]. Eur Radiol, 2019, 29( 2): 654- 662. DOI: 10.1007/s00330-018-5617-6. |
| [5] |
ABDO J, CORNELL DL, MITTAL SK, et al. Immunotherapy plus cryotherapy: potential augmented abscopal effect for advanced cancers[J]. Front Oncol, 2018, 8: 85. DOI: 10.3389/fonc.2018.00085. |
| [6] |
CHEN LT, MARTINELLI E, CHENG AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO[J]. Ann Oncol, 2020, 31( 3): 334- 351. DOI: 10.1016/j.annonc.2019.12.001. |
| [7] |
BENSON AB, D’ANGELICA MI, ABBOTT DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19( 5): 541- 565. DOI: 10.6004/jnccn.2021.0022. |
| [8] |
SCHREIBER RD, OLD LJ, SMYTH MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion[J]. Science, 2011, 331( 6024): 1565- 1570. DOI: 10.1126/science.1203486. |
| [9] |
CHENG AL, QIN S, IKEDA M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma[J]. J Hepatol, 2022, 76( 4): 862- 873. DOI: 10.1016/j.jhep.2021.11.030. |
| [10] |
PENG Z, FAN W, ZHU B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A Phase Ⅲ, Randomized Clinical Trial-LAUNCH)[J]. J Clin Oncol, 2023, 41( 1): 117- 127. DOI: 10.1200/JCO.22.00392. |
| [11] |
KUDO M, UESHIMA K, IKEDA M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation(TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial[J]. Gut, 2020, 69( 8): 1492- 1501. DOI: 10.1136/gutjnl-2019-318934. |
| [12] |
DUFFY AG, ULAHANNAN SV, MAKOROVA-RUSHER O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma[J]. J Hepatol, 2017, 66( 3): 545- 551. DOI: 10.1016/j.jhep.2016.10.029. |
| [13] |
NIU LZ, LI JL, ZENG JY, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer[J]. World J Gastroenterol, 2013, 19( 22): 3473- 3480. DOI: 10.3748/wjg.v19.i22.3473. |
| [14] |
YAKKALA C, DENYS A, KANDALAFT L, et al. Cryoablation and immunotherapy of cancer[J]. Curr Opin Biotechnol, 2020, 65: 60- 64. DOI: 10.1016/j.copbio.2020.01.006. |
| [15] |
TAN J, LIU T, FAN W, et al. Anti-PD-L1 antibody enhances curative effect of cryoablation via antibody-dependent cell-mediated cytotoxicity mediating PD-L1highCD11b+ cells elimination in hepatocellular carcinoma[J]. Acta Pharm Sin B, 2023, 13( 2): 632- 647. DOI: 10.1016/j.apsb.2022.08.006. |
| [16] |
KWAK K, YU B, LEWANDOWSKI RJ, et al. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation[J]. Theranostics, 2022, 12( 5): 2175- 2204. DOI: 10.7150/thno.67530. |
| [17] |
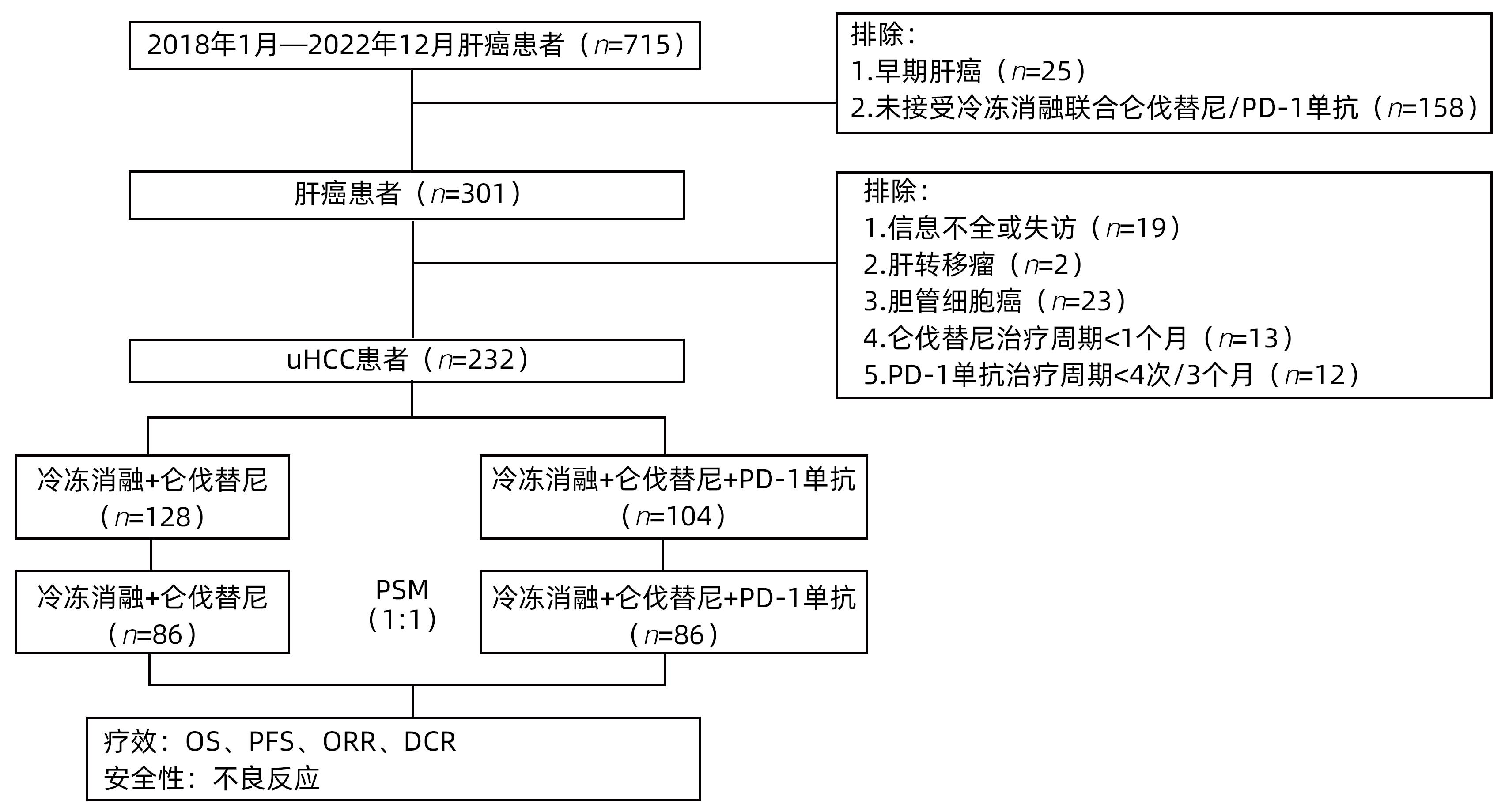
|
| [18] |
LU M, ZHANG X, GAO X, et al. Lenvatinib enhances T cell immunity and the efficacy of adoptive chimeric antigen receptor-modified T cells by decreasing myeloid-derived suppressor cells in cancer[J]. Pharmacol Res, 2021, 174: 105829. DOI: 10.1016/j.phrs.2021.105829. |
| [19] |
YANG XR, SUN HC, XIE Q, et al. Chinese expert guidance on overall application of lenvatinib in hepatocellular carcinoma[J]. Chin J Dig Surg, 2023, 22( 2): 167- 180. DOI: 10.3760/cma.j.cn115610-20230201-00035. |
| [20] |
YANG Y, LU Y, WANG C, et al. Cryotherapy is associated with improved clinical outcomes of Sorafenib therapy for advanced hepatocellular carcinoma[J]. Cell Biochem Biophys, 2012, 63( 2): 159- 169. DOI: 10.1007/s12013-012-9353-2. |
| [21] |
WANG YY, YANG X, WANG YC, et al. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma[J]. World J Gastroenterol, 2023, 29( 10): 1614- 1626. DOI: 10.3748/wjg.v29.i10.1614. |
| [22] |
CAO F, YANG Y, SI T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study[J]. Front Oncol, 2021, 11: 783480. DOI: 10.3389/fonc.2021.783480. |
| [23] |
CAI M, HUANG W, HUANG J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study[J]. Front Immunol, 2022, 13: 848387. DOI: 10.3389/fimmu.2022.848387. |
| [24] |
PARK JW, KIM YJ, KIM DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase Ⅲ STAH trial[J]. J Hepatol, 2019, 70( 4): 684- 691. DOI: 10.1016/j.jhep.2018.11.029. |
| [25] |
MARINELLI B, KIM E, D’ALESSIO A, et al. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study[J]. J Immunother Cancer, 2022, 10( 6). DOI: 10.1136/jitc-2021-004205. |
| [26] |
YUAN Y, HE W, YANG Z, et al. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study[J]. Int J Surg, 2023, 109( 5): 1222- 1230. DOI: 10.1097/JS9.0000000000000256. |
| [27] |
CUI W, FAN W, HUANG K, et al. Large hepatocellular carcinomas: treatment with transarterial chemoembolization alone or in combination with percutaneous cryoablation[J]. Int J Hyperthermia, 2018, 35( 1): 239- 245. DOI: 10.1080/02656736.2018.1493235. |
| [28] |
KUDO M, FINN RS, QIN S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391( 10126): 1163- 1173. DOI: 10.1016/S0140-6736(18)30207-1. |







 DownLoad:
DownLoad:




