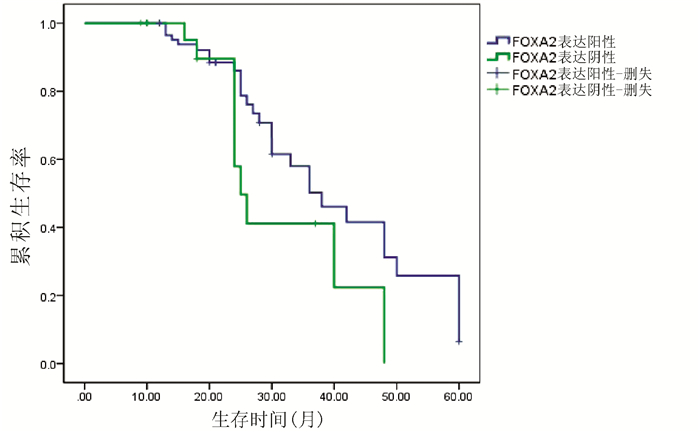
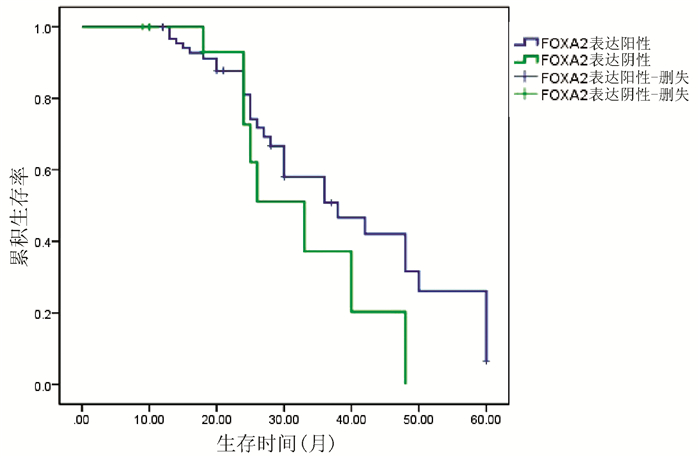
| [1] |
Bureau of Medical AdministrationNational Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. |
| [2] |
CHEN W, ZHENG R, BAADE PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin. 2016, 66(2): 115-32. DOI: 10.3322/caac.21338. |
| [3] |
SIA D, VILLANUEVA A, FRIEDMAN SL, et al. Liver cancer cell of origin, molecular class, and effects on patient prognosis[J]. Gastroenterology, 2017, 152(4): 745-761. DOI: 10.1053/j.gastro.2016.11.048. |
| [4] |
HAN JH, KIM DG, NA GH, et al. Evaluation of prognostic factors on recurrence after curative resections for hepatocellular carcinoma[J]. World J Gastroenterol, 2014, 20(45): 17132-17140. DOI: 10.3748/wjg.v20.i45.17132. |
| [5] |
BOULIN M, DELHOM E, PIERREDON-FOULONGNE MA, et al. Transarterial chemoembolization for hepatocellular carcinoma: An old method, now flavor of the day[J]. Diagn Interv Imaging, 2015, 96(6): 607-615. DOI: 10.1016/j.diii.2015.04.005. |
| [6] |
YANG J, YAN L, WANG W. Current status of multimodal & combination therapy for hepatocellular carcinoma[J]. Indian J Med Res, 2012, 136(3): 391-403.
|
| [7] |
WANG J, LI W, ZHAO Y, et al. Members of FOX family could be drug targets of cancers[J]. Pharmacol Ther, 2018, 181: 183-196. DOI: 10.1016/j.pharmthera.2017.08.003. |
| [8] |
KATOH M, IGARASHI M, FUKUDA H, et al. Cancer genetics and genomics of human FOX family genes[J]. Cancer Lett, 2013, 328(2): 198-206. DOI: 10.1016/j.canlet.2012.09.017. |
| [9] |
|
| [10] |
MYATT SS, LAM EW. The emerging roles of forkhead box (Fox) proteins in cancer[J]. Nat Rev Cancer, 2007, 7(11): 847-859. DOI: 10.1038/nrc2223. |
| [11] |
LIU J, YU Z, XIAO Y, et al. Coordination of FOXA2 and SIRT6 suppresses the hepatocellular carcinoma progression through ZEB2 inhibition[J]. Cancer Manag Res, 2018, 10: 391-402. DOI: 10.2147/CMAR.S150552. |
| [12] |
ZHANG H, TANG QF, SUN MY, et al. ARHGAP9 suppresses the migration and invasion of hepatocellular carcinoma cells through up-regulating FOXJ2/E-cadherin[J]. Cell Death Dis, 2018, 9(9): 916. DOI: 10.1038/s41419-018-0976-0. |
| [13] |
SHAN Y, CHANG T, SHI S, et al. Foxj2 overexpression is associated with poor prognosis, progression, and metastasis in nasopharyngeal carcinoma[J]. Onco Targets Ther, 2017, 10: 3733-3741. DOI: 10.2147/OTT.S134915. |
| [14] |
TSUCHIYA N, SAWADA Y, ENDO I, et al. Biomarkers for the early diagnosis of hepatocellular carcinoma[J]. World J Gastroenterol, 2015, 21(37): 10573-10583. DOI: 10.3748/wjg.v21.i37.10573. |
| [15] |
LI H, CHEN D, ZHANG J. Analysis of intron sequence features associated with transcriptional regulation in human genes[J]. PLoS One, 2012, 7(10): e46784. DOI: 10.1371/journal.pone.0046784. |
| [16] |
LEE TI, YOUNG RA. Transcriptional regulation and its misregulation in disease[J]. Cell, 2013, 152(6): 1237-1251. DOI: 10.1016/j.cell.2013.02.014. |
| [17] |
LEHMANN OJ, SOWDEN JC, CARLSSON P, et al. Fox's in development and disease[J]. Trends Genet, 2003, 19(6): 339-344. DOI: 10.1016/S0168-9525(03)00111-2. |
| [18] |
HANNENHALLI S, KAESTNER KH. The evolution of Fox genes and their role in development and disease[J]. Nat Rev Genet, 2009, 10(4): 233-240. DOI: 10.1038/nrg2523. |
| [19] |
JIN Y, LIANG Z, LOU H. The emerging roles of fox family transcription factors in chromosome replication, organization, and genome stability[J]. Cells, 2020, 9(1): 258. DOI: 10.3390/cells9010258. |
| [20] |
BENAYOUN BA, CABURET S, VEITIA RA. Forkhead transcription factors: Key players in health and disease[J]. Trends Genet, 2011, 27(6): 224-232. DOI: 10.1016/j.tig.2011.03.003. |
| [21] |
LI J, DANTAS MACHADO AC, GUO M, et al. Structure of the forkhead domain of FOXA2 bound to a complete DNA consensus site[J]. Biochemistry, 2017, 56(29): 3745-3753. DOI: 10.1021/acs.biochem.7b00211. |
| [22] |
LIU J, YU Z, XIAO Y, et al. Coordination of FOXA2 and SIRT6 suppresses the hepatocellular carcinoma progression through ZEB2 inhibition[J]. Cancer Manag Res, 2018, 10: 391-402. DOI: 10.2147/CMAR.S150552. |
| [23] |
LI Z, TUTEJA G, SCHUG J, et al. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer[J]. Cell, 2012, 148(1-2): 72-83. DOI: 10.1016/j.cell.2011.11.026. |
| [24] |
WANG W, YAO LJ, SHEN W, et al. FOXA2 alleviates CCl 4-induced liver fibrosis by protecting hepatocytes in mice[J]. Sci Rep, 2017, 7(1): 15532. DOI: 10.1038/s41598-017-15831-6. |
| [25] |
YAMAMURA N, FUGO K, KISHIMOTO T. Forkhead box protein A2, a pioneer factor for hepatogenesis, is involved in the expression of hepatic phenotype of alpha-fetoprotein-producing adenocarcinoma[J]. Pathol Res Pract, 2017, 213(9): 1082-1088. DOI: 10.1016/j.prp.2017.07.024. |
| [26] |
QI J, NAKAYAMA K, CARDIFF RD, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors[J]. Cancer Cell, 2010, 18(1): 23-38. DOI: 10.1016/j.ccr.2010.05.024. |
| [27] |
SMITH B, NEFF R, COHN DE, et al. The mutational spectrum of FOXA2 in endometrioid endometrial cancer points to a tumor suppressor role[J]. Gynecol Oncol, 2016, 143(2): 398-405. DOI: 10.1016/j.ygyno.2016.08.237. |
| [28] |
BASSERES DS, D'ALÒ F, YEAP BY, et al. Frequent downregulation of the transcription factor Foxa2 in lung cancer through epigenetic silencing[J]. Lung Cancer, 2012, 77(1): 31-37. DOI: 10.1016/j.lungcan.2012.01.011. |
| [29] |
LI C, LU S, SHI Y. MicroRNA-187 promotes growth and metastasis of gastric cancer by inhibiting FOXA2[J]. Oncol Rep, 2017, 37(3): 1747-1755. DOI: 10.3892/or.2017.5370. |
| [30] |
DING B, LIANG H, GAO M, et al. Forkhead Box A2 (FOXA2) inhibits invasion and tumorigenesis in glioma cells[J]. Oncol Res, 2017, 25(5): 701-708. DOI: 10.3727/096504016X14772378087005. |
| [31] |
WANG J, ZHU CP, HU PF, et al. FOXA2 suppresses the metastasis of hepatocellular carcinoma partially through matrix metalloproteinase-9 inhibition[J]. Carcinogenesis, 2014, 35(11): 2576-2583. DOI: 10.1093/carcin/bgu180. |
| [32] |
LIU J, YU Z, XIAO Y, et al. Coordination of FOXA2 and SIRT6 suppresses the hepatocellular carcinoma progression through ZEB2 inhibition[J]. Cancer Manag Res, 2018, 10: 391-402. DOI: 10.2147/CMAR.S150552. |
| [33] |
PÉREZ-SÁNCHEZ C, ARIAS-DE-LA-FUENTE C, GÓMEZ-FERRERíA MA, et al. FHX. L and FHX. S, two isoforms of the human fork-head factor FHX (FOXJ2) with differential activity[J]. J Mol Biol, 2000, 301(4): 795-806. DOI: 10.1006/jmbi.2000.3999. |
| [34] |
GRANADINO B, ARIAS-DE-LA-FUENTE C, PÉREZ-SÁNCHEZ C, et al. Fhx (Foxj2) expression is activated during spermatogenesis and very early in embryonic development[J]. Mech Dev, 2000, 97(1-2): 157-160. DOI: 10.1016/s0925-4773(00)00410-x. |
| [35] |
|
| [36] |
MARTÍN-DE-LARA F, SÁNCHEZ-APARICIO P, ARIAS DE LA FUENTE C, et al. Biological effects of FoxJ2 over-expression[J]. Transgenic Res, 2008, 17(6): 1131-1141. DOI: 10.1007/s11248-008-9214-3. |
| [37] |
QIU X, JI B, YANG L, et al. The role of FoxJ2 in the migration of human glioma cells[J]. Pathol Res Pract, 2015, 211(5): 389-397. DOI: 10.1016/j.prp.2015.01.005. |
| [38] |
YANG Q, CAO X, TAO G, et al. Effects of FOXJ2 on TGF-β1-induced epithelial-mesenchymal transition through Notch signaling pathway in non-small lung cancer[J]. Cell Biol Int, 2017, 41(1): 79-83. DOI: 10.1002/cbin.10680. |
| [39] |
WANG Y, YANG S, NI Q, et al. Overexpression of forkhead box J2 can decrease the migration of breast cancer cells[J]. J Cell Biochem, 2012, 113(8): 2729-2737. DOI: 10.1002/jcb.24146. |
| [40] |
ZHANG Z, MENG G, WANG L, et al. The prognostic role and reduced expression of FOXJ2 in human hepatocellular carcinoma[J]. Mol Med Rep, 2016, 14(1): 254-262. DOI: 10.3892/mmr.2016.5261. |










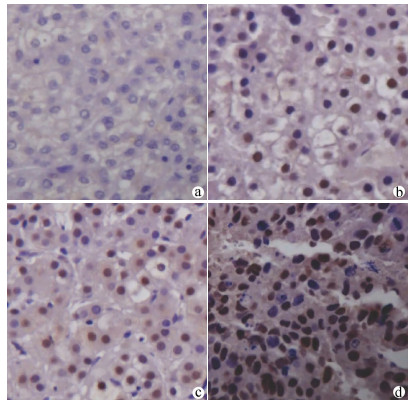




 DownLoad:
DownLoad: