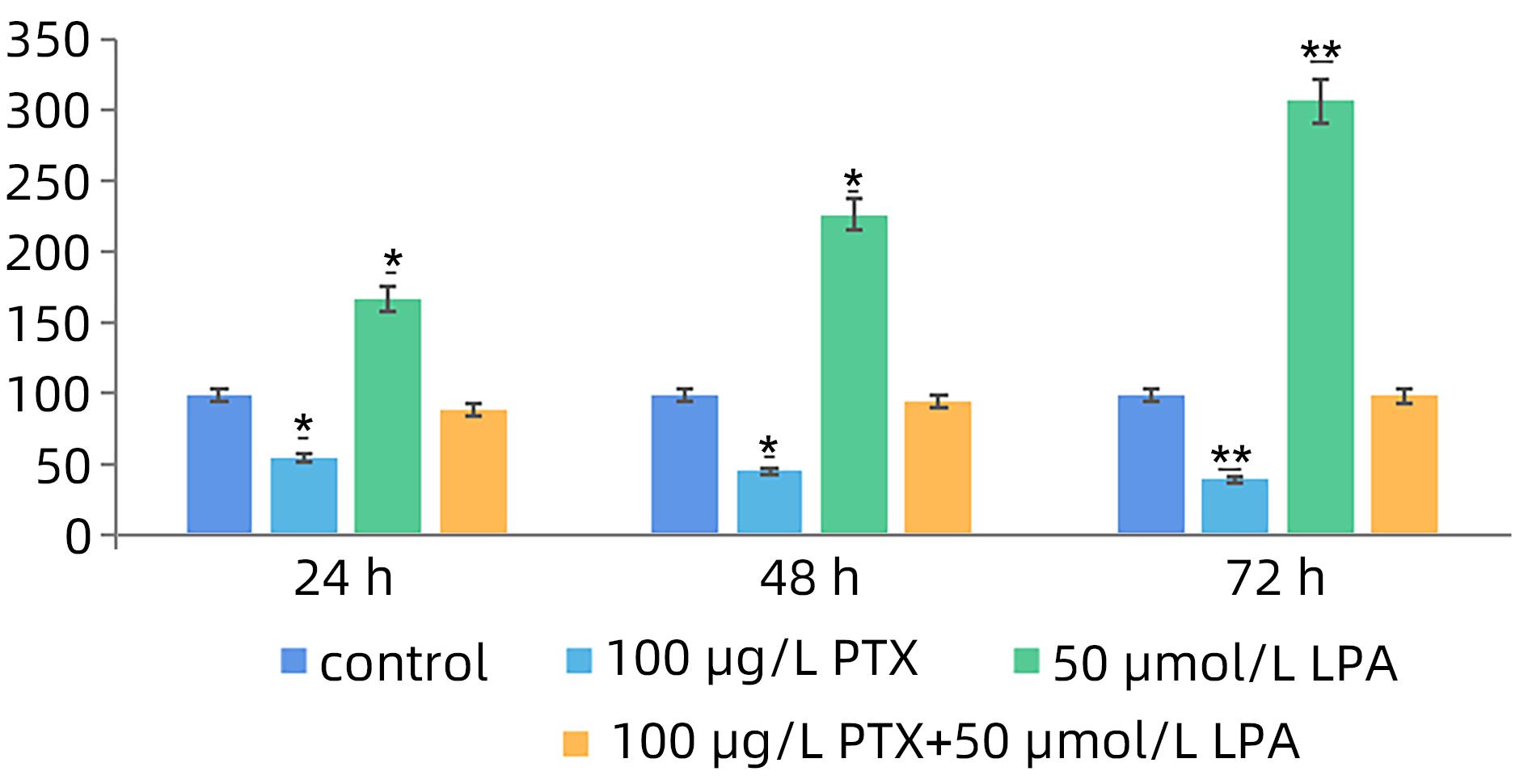
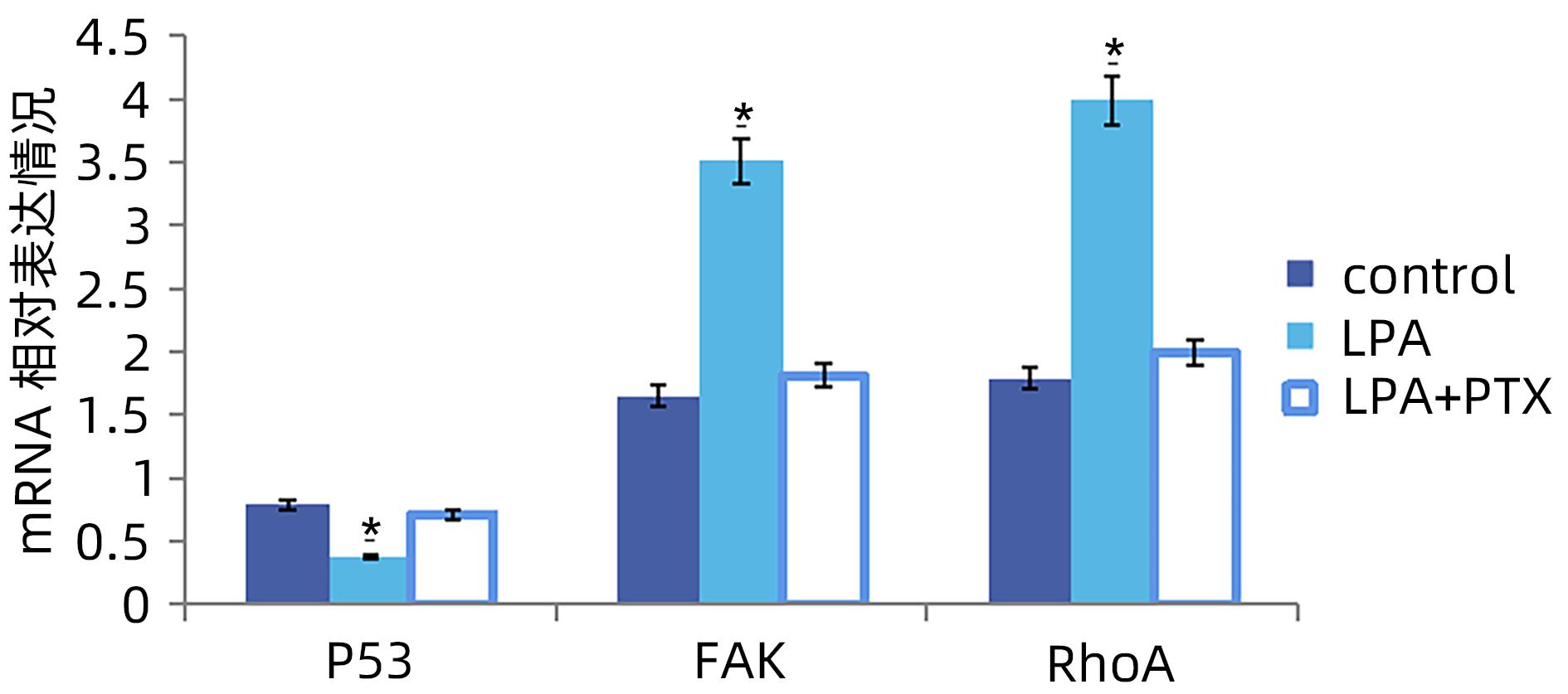
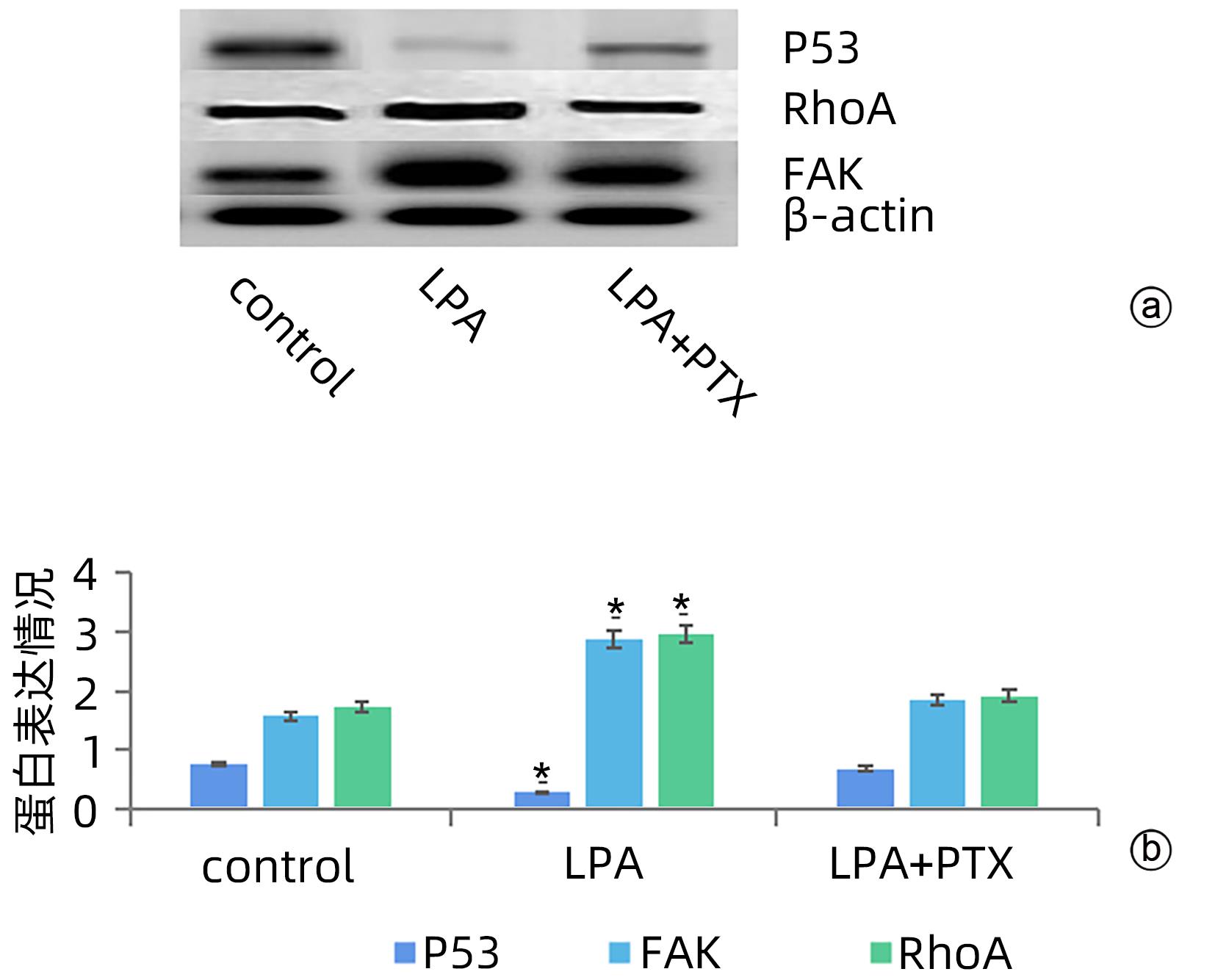
| [1] |
LI X, RAMADORI P, PFISTER D, et al. The immunological and metabolic landscape in primary and metastatic liver cancer[J]. Nat Rev Cancer, 2021, 21( 9): 541- 557. DOI: 10.1038/s41568-021-00383-9. |
| [2] |
XU Z, LIU Y, LIANG F. Clinical efficacy of Danshen Chuanxiongqin Injection and its effect on serum levels of LPA,Hcy and MCP-1 in patients with ischemic stroke[J]. J Changchun Univ Chin Med, 2021, 37( 1): 84- 87. DOI: 10.13463/j.cnki.cczyy.2021.01.023. |
| [3] |
AIELLO S, CASIRAGHI F. Lysophosphatidic acid: Promoter of cancer progression and of tumor microenvironment development. A promising target for anticancer therapies?[J]. Cells, 2021, 10( 6): 1390. DOI: 10.3390/cells10061390. |
| [4] |
RAY R, JANGDE N, SINGH SK, et al. Lysophosphatidic acid-RAGE axis promotes lung and mammary oncogenesis via protein kinase B and regulating tumor microenvironment[J]. Cell Commun Signal, 2020, 18( 1): 170. DOI: 10.1186/s12964-020-00666-y. |
| [5] |
AMARAL RF, GERALDO LHM, EINICKER-LAMAS M, et al. Microglial lysophosphatidic acid promotes glioblastoma proliferation and migration via LPA 1 receptor[J]. J Neurochem, 2021, 156( 4): 499- 512. DOI: 10.1111/jnc.15097. |
| [6] |
KLYMENKO Y, BOS B, CAMPBELL L, et al. Lysophosphatidic acid modulates ovarian cancer multicellular aggregate assembly and metastatic dissemination[J]. Sci Rep, 2020, 10( 1): 10877. DOI: 10.1038/s41598-020-67565-7. |
| [7] |
GNOCCHI D, CAVALLUZZI MM, MANGIATORDI GF, et al. Xanthenylacetic acid derivatives effectively target lysophosphatidic acid receptor 6 to inhibit hepatocellular carcinoma cell growth[J]. ChemMedChem, 2021, 16( 13): 2121- 2129. DOI: 10.1002/cmdc.202100032. |
| [8] |
DEHGHAN M, SHAHBAZI S, SALEHNIA M. Effect of lysophosphatidic acid on the vascular endothelial growth factor expression in autotransplanted mouse ovaries encapsulated in sodium alginate[J]. J Family Reprod Health, 2021, 15( 2): 91- 98. DOI: 10.18502/jfrh.v15i2.6449. |
| [9] |
XIE Y, WANG XC, WU XW, et al. Lysophosphatidic acid receptor 4 regulates osteogenic and adipogenic differentiation of progenitor cells via inactivation of RhoA/ROCK1/β-catenin signaling[J]. Stem Cells, 2020, 38( 3): 451- 463. DOI: 10.1002/stem.3128. |
| [10] |
MINAMI K, UEDA N, ISHIMOTO K, et al. Cooperation of G12/13 and Gi proteins via lysophosphatidic acid receptor-2(LPA 2) signaling enhances cancer cell survival to cisplatin[J]. Biochem Biophys Res Commun, 2020, 532( 3): 427- 432. DOI: 10.1016/j.bbrc.2020.08.087. |
| [11] |
LEE SC, LIN KH, BALOGH A, et al. Dysregulation of lysophospholipid signaling by p53 in malignant cells and the tumor microenvironment[J]. Cell Signal, 2021, 78: 109850. DOI: 10.1016/j.cellsig.2020.109850. |
| [12] |
ZHANG YZ, SHI HB, CHEN Y. Biological functions of apoptosis stimulating protein of p53 2 and its role in liver diseases[J]. J Clin Hepatol, 2018, 34( 11): 2443- 2447. DOI: 10.3969/j.issn.1001-5256.2018.11.039. |
| [13] |
HUANG CC, TSENG TT, LIU SC, et al. S1P increases VEGF production in osteoblasts and facilitates endothelial progenitor cell angiogenesis by inhibiting miR-16-5p expression via the c-src/FAK signaling pathway in rheumatoid arthritis[J]. Cells, 2021, 10( 8): 2168. DOI: 10.3390/cells10082168. |
| [14] |
WEI YH, WANG YF, LIU NB, et al. A FAK inhibitor boosts anti-PD1 immunotherapy in a hepatocellular carcinoma mouse model[J]. Front Pharmacol, 2022, 12: 820446. DOI: 10.3389/fphar.2021.820446. |
| [15] |
LIAO Y, LIU L, YANG JY, et al. ATX/LPA axis regulates FAK activation, cell proliferation, apoptosis, and motility in human pancreatic cancer cells[J]. Vitro Cell Dev Biol Anim, 2022, 58( 4): 307- 315. DOI: 10.1007/s11626-022-00660-3. |
| [16] |
SUMITOMO A, SIRIWACH R, THUMKEO D, et al. LPA induces keratinocyte differentiation and promotes skin barrier function through the LPAR1/LPAR5-RHO-ROCK-SRF axis[J]. J Invest Dermatol, 2019, 139( 5): 1010- 1022. DOI: 10.1016/j.jid.2018.10.034. |
| [17] |
KIM D, KIM HJ, BAEK JO, et al. Lysophosphatidic acid mediates imiquimod-induced psoriasis-like symptoms by promoting keratinocyte proliferation through LPAR1/ROCK2/PI3K/AKT signaling pathway[J]. Int J Mol Sci, 2021, 22( 19): 10777. DOI: 10.3390/ijms221910777. |
| [18] |
NAKAJIMA K, OKA S, TANIKAWA T, et al. Lysophosphatidylinositol induced morphological changes and stress fiber formation through the GPR55-RhoA-ROCK pathway[J]. Int J Mol Sci, 2022, 23( 18): 10932. DOI: 10.3390/ijms231810932. |
| [19] |
INABA A, HARADA H, IKEZAKI S, et al. LPA 6-RhoA signals regulate junctional complexes for polarity and morphology establishment of maturation stage ameloblasts[J]. J Oral Biosci, 2022, 64( 1): 85- 92. DOI: 10.1016/j.job.2022.01.004. |
| [20] |
BUTERA A, ROY M, ZAMPIERI C, et al. p53-driven lipidome influences non-cell-autonomous lysophospholipids in pancreatic cancer[J]. Biol Direct, 2022, 17( 1): 6. DOI: 10.1186/s13062-022-00319-9. |








 本站查看
本站查看

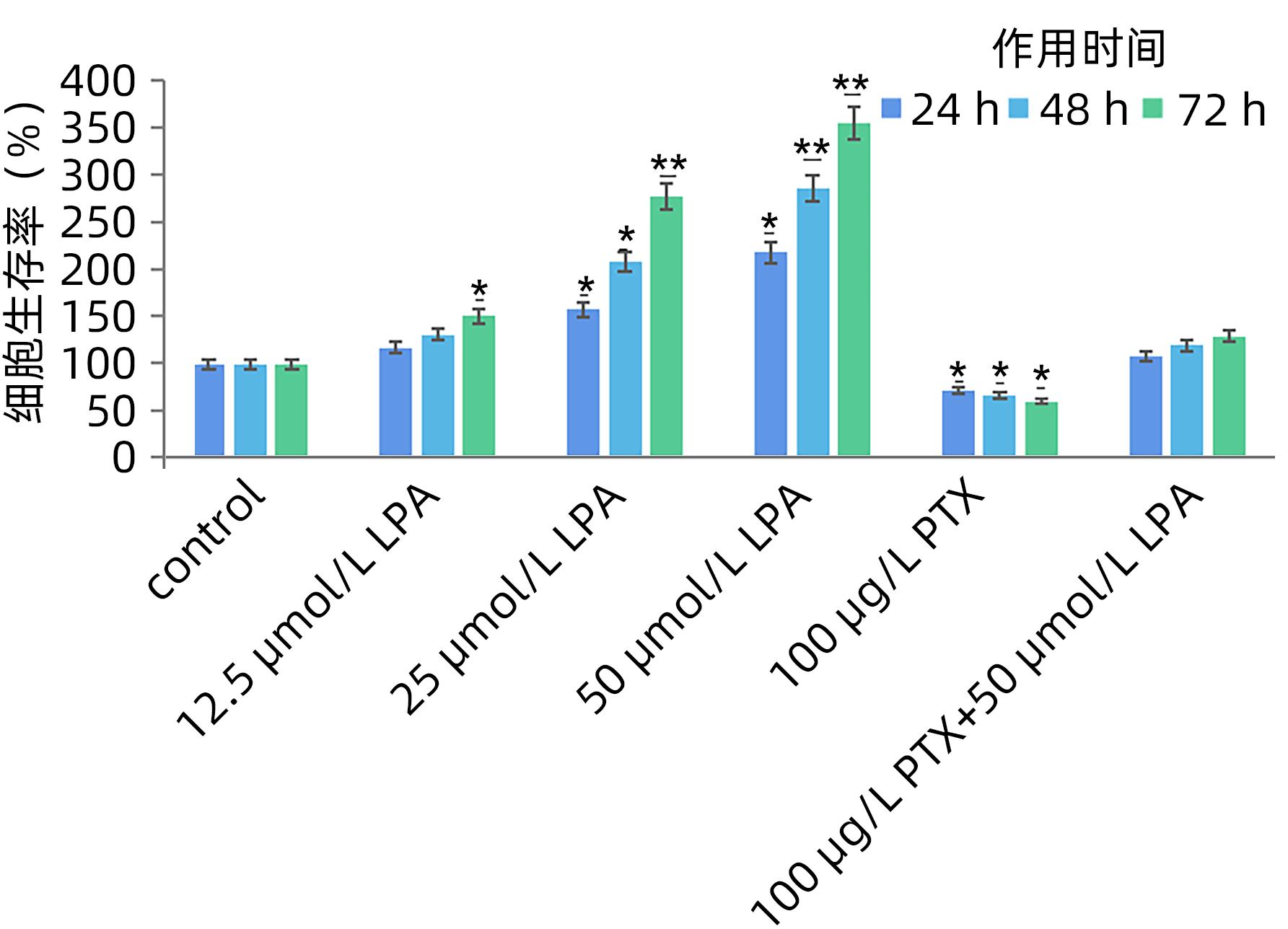




 DownLoad:
DownLoad: